Guidelines for the management of emergencies and critical illness in pediatric and adult patients with sickle cell disease
- PMID: 40439782
- PMCID: PMC12123041
- DOI: 10.1186/s13613-025-01479-3
Guidelines for the management of emergencies and critical illness in pediatric and adult patients with sickle cell disease
Abstract
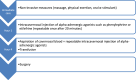
Forty-two questions were evaluated concerning management of emergencies and critical illnesses in paediatric and adult patients with sickle cell disease. The assessment covered the following areas: patient referral, vaso-occlusive crisis, acute chest syndrome, transfusion therapy, and priapism. The patient referral category included guidelines for admission to intensive care unit and management at specialized reference centers. The vaso-occlusive crisis topic encompassed pain management, hydration, incentive spirometry, and target oxygen saturation levels. For acute chest syndrome, the focus areas included imaging techniques such as lung ultrasound, computed tomography scans, and echocardiography; treatment with systemic corticosteroids; non-invasive ventilation; prophylactic and therapeutic anticoagulation; and procalcitonin and antibiotic therapy. The section on transfusion therapy addressed indications and methods of transfusion, as well as the diagnosis and prediction of delayed hemolytic transfusion reactions. A total of 45 recommendations were proposed, including 14 specific to adults, 13 specific to pediatrics, and 18 applicable to both adults and children, along with three therapeutic algorithms. The Grade of Recommendation Assessment, Development, and Evaluation (GRADE) methodology was adhered to throughout the process. Sixteen recommendations were based on a low level of evidence (GRADE 2+ or 2-), while 26 were based on evidence that could not be classified under the GRADE system and were therefore considered expert opinions. Finally, for three aspects of sickle cell disease management, the experts concluded that no reliable recommendations could be made based on the current state of knowledge. The recommendations and therapeutic algorithms received strong agreement from the experts.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Consent for publication: Not applicable. Competing interests: MEKONTSO DESSAP Armand reports grants and fees from Addmedica, Fisher & Paykel Healthcare, and Orkyn. RAZAZI Keyvan has received lecture fees from MSD, Shionogi, and a travel grant from Pfizer outside the submitted work. PIRENNE France has received honoraria from GRIFOLS. ARLET Jean-Benoît declares that he has received research funding from Theravia, GBT-Pfizer, Novartis, and Vertex, and has been supported at scientific conferences by Novartis France, GBT. AUBRON Cécile has received lecture fees from CSL-VIFOR, Cereus and MSD. BAUDIN Florent reports a relationship with Dräger Medical, Fisher & Paykel Healthcare, Sedana Medical that includes non-financial support and travel reimbursement. ELENGA Narcisse has received consultant fees from THERAVIA. GENDREAU Ségolène reports support from Pharma Dom for attending a meeting, outside the submitted work. HABIBI Anoosha has received fees as a consulted for Novartis, GBT-Pfizer and Theravia, and attended conferences sponsored by Novartis and GBT-Pfizer. PESCHANSKI Nicolas reports fees from Fisher&Paykel Healthcare SAS. PONDARRE Corinne reports honoraria for Novartis and expert consultancy for Addmedica, Global Blood Therapeutics and Pfizer. MAITRE Bernard reports research funding from Ikaria (ex iNOTherapeutics) and Leo Pharma (clinical trials in acute chest syndrome). THURET Isabelle reports honoraria from Pfizer and Vertex as menber of advisory board. Other authors declared no competing interest.
Figures


References
-
- Cecchini J, Lionnet F, Djibré M, Parrot A, Stojanovic K, Girot R, et al. Outcomes of adult patients with sickle cell disease admitted to the ICU: a case series*. Crit Care Med. 2014;42:1629–39. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

