VSV-CHIKV activates antitumor immunity by inducing pyroptosis in a melanoma model
- PMID: 40439822
- PMCID: PMC12122967
- DOI: 10.1007/s12672-025-02788-6
VSV-CHIKV activates antitumor immunity by inducing pyroptosis in a melanoma model
Abstract
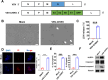
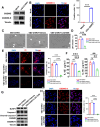
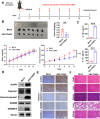
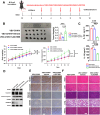
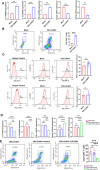
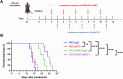
Melanoma is the most dangerous skin cancer due to its difficulty in treatment, high recurrence rate and metastatic ability. As a vector for oncolytic viruses (OVs), vesicular stomatitis virus (VSV) has been shown to be effective against malignant melanoma. However, the glycoprotein G protein of VSV has potential neurotoxicity. It has been shown that replacing glycoprotein G with E3-E2-6K-E1 of chikungunya virus (CHIKV) reduces its neurotoxicity and targets gliomas. Therefore, the aim of this study was to investigate the oncolytic effect of recombinant VSV-CHIKV on melanoma and the underlying mechanism. In this study, we found that recombinant VSV-CHIKV triggered GSDMD-mediated melanoma cell pyroptosis. Importantly, the NLRP3/Caspase-1/GSDMD axis was activated after VSV-CHIKV infection in melanoma cell lines and in a xenograft mouse model. Inhibition of GSDMD blocked cell pyroptosis, antitumor immunity and the tumor response in response to VSV-CHIKV treatment, suggesting that VSV-CHIKV act through the GSDMD pathway. VSV-CHIKV-triggered GSDMD-mediated tumor pyroptosis recruited cytotoxic T lymphocytes (CTLs) into the tumor microenvironment, which was accompanied by the release of inflammatory mediators. This remodeled the tumor microenvironment and turned immunologically "cold" tumors into "hot" tumors, thereby sensitized these tumors to checkpoint blockade. Finally, the combination therapy of VSV-CHIKV and an immune checkpoint inhibitor (anti-PD-1) prolonged the survival of mice. In conclusion, the VSV-CHIKV strategy is an attractive biologic therapy against melanoma.
Keywords: Melanoma; Oncolytic virus; Pyroptosis; Vesicular stomatitis virus.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethical approval: Animal experiments were approved by the Animal care & Welfare Committee, School of Stomatology, Fourth Military Medical University (kq-2022–020) and followed the ARRIVE guidelines. All methods were performed in accordance with relevant guidelines and regulations. Consent to participate: Not applicable. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures

Similar articles
-
Vesicular stomatitis virus sensitizes immunologically cold tumors to checkpoint blockade by inducing pyroptosis.Biochim Biophys Acta Mol Basis Dis. 2022 Dec 1;1868(12):166538. doi: 10.1016/j.bbadis.2022.166538. Epub 2022 Sep 9. Biochim Biophys Acta Mol Basis Dis. 2022. PMID: 36096276
-
Chikungunya-vesicular stomatitis chimeric virus targets and eliminates brain tumors.Virology. 2018 Sep;522:244-259. doi: 10.1016/j.virol.2018.06.018. Epub 2018 Jul 26. Virology. 2018. PMID: 30055515 Free PMC article.
-
Chikungunya, Influenza, Nipah, and Semliki Forest Chimeric Viruses with Vesicular Stomatitis Virus: Actions in the Brain.J Virol. 2017 Feb 28;91(6):e02154-16. doi: 10.1128/JVI.02154-16. Print 2017 Mar 15. J Virol. 2017. PMID: 28077641 Free PMC article.
-
Phase I study of VSV-GP (BI 1831169) as monotherapy or combined with ezabenlimab in advanced and refractory solid tumors.Future Oncol. 2022 Aug;18(24):2627-2638. doi: 10.2217/fon-2022-0439. Epub 2022 Jun 14. Future Oncol. 2022. PMID: 35699077 Review.
-
Immunovirotherapy Based on Recombinant Vesicular Stomatitis Virus: Where Are We?Front Immunol. 2022 Jun 28;13:898631. doi: 10.3389/fimmu.2022.898631. eCollection 2022. Front Immunol. 2022. PMID: 35837384 Free PMC article. Review.
References
-
- Long GV, Swetter SM, Menzies AM, Gershenwald JE, Scolyer RA. Cutaneous melanoma. Lancet. 2023 - PubMed
-
- Centeno PP, Pavet V, Marais R. The journey from melanocytes to melanoma. Nat Rev Cancer. 2023;23(6):372–90. - PubMed
-
- Kalaora S, Nagler A, Wargo JA, Samuels Y. Mechanisms of immune activation and regulation: lessons from melanoma. Nat Rev Cancer. 2022;22(4):195–207. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
