New Definition of Light Chain Monoclonal Gammopathy of Undetermined Significance
- PMID: 40440024
- PMCID: PMC12123532
- DOI: 10.1001/jamaoncol.2025.1285
New Definition of Light Chain Monoclonal Gammopathy of Undetermined Significance
Abstract
Importance: Recent studies suggest standard reference intervals for serum free light chains (FLC) are inaccurate and that this problem can only be partially remedied by using separate reference intervals for individuals with impaired kidney function. This decreases the utility of FLC testing in the clinical evaluation and follow-up of plasma cell disorders, particularly affecting the diagnosis of light chain (LC) monoclonal gammopathy of undetermined significance (MGUS).
Objective: To evaluate the distribution of serum FLC and FLC ratios in individuals with preserved kidney function and to propose revised reference intervals and a new definition of LC-MGUS.
Design, setting, and participants: The Iceland Screens, Treats or Prevents Multiple Myeloma (iStopMM) study is a nationwide prospective study of 75 422 participants (more than 50% of the Icelandic population) 40 years and older who were screened for MGUS. Data were collected from September 2016 to May 2023, and data were analyzed from June 2023 to May 2024.
Exposure: Samples were analyzed by serum protein electrophoresis and immunofixation electrophoresis and FLC assay. Participants were actively followed up for progression.
Main outcomes and measures: The rate of abnormal FLC results using standard reference intervals was assessed, and revised age-stratified 99% reference intervals were calculated using nonparametric regression. The prevalence of LC-MGUS based on standard and revised reference intervals was evaluated along with progression to lymphoproliferative disorders.
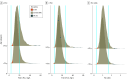
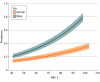
Results: In total, 41 882 participants met inclusion criteria; a total of 23 786 (56.8%) were female, and the median (IQR) age was 60 (52-68) years. Using standard FLC reference intervals, 7316 κ FLC (17.5%), 1668 λ FLC (4.0%), and 1543 FLC ratios (3.7%) were abnormal. Revised reference intervals were calculated for those younger than 70 years (κ FLC, 6.3-39.0 mg/L; λ FLC, 5.9-36.7 mg/L; FLC ratio, 0.44-2.16) and 70 years or older (κ FLC, 7.0-55.8 mg/L; λ FLC, 6.4-48.0 mg/L; FLC ratio, 0.46-2.59). The prevalence of LC-MGUS was 1.54% (95% CI, 1.46-1.63) using standard intervals and 0.27% (95% CI, 0.23-0.30) using the revised intervals, yielding a decrease of 82%. None of the 1006 persons meeting LC-MGUS criteria based on standard intervals but not based on revised intervals progressed to a lymphoproliferative disorder during a median (range) follow-up of 4.6 (2.5-6.7) years.
Conclusions and relevance: In this study, a new definition of LC-MGUS based on revised, more accurate FLC reference intervals decreased the false-positive rate of FLC testing by 82%.
Conflict of interest statement
Figures
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

