The proofreading mechanism of the human leading-strand DNA polymerase ε holoenzyme
- PMID: 40440070
- PMCID: PMC12146725
- DOI: 10.1073/pnas.2507232122
The proofreading mechanism of the human leading-strand DNA polymerase ε holoenzyme
Erratum in
-
Correction for Wang et al., The proofreading mechanism of the human leading-strand DNA polymerase ε holoenzyme.Proc Natl Acad Sci U S A. 2025 Jul 29;122(30):e2516665122. doi: 10.1073/pnas.2516665122. Epub 2025 Jul 23. Proc Natl Acad Sci U S A. 2025. PMID: 40699934 Free PMC article. No abstract available.
Abstract
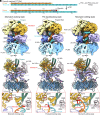
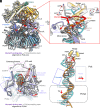
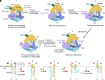
The eukaryotic leading-strand DNA polymerase ε (Polε) is a dual-function enzyme with a proofreading 3'-5' exonuclease (exo) site located 40 Å from the DNA synthesizing pol site. Errors in Polε proofreading can cause various mutations, including C-to-G transversions, the most prevalent mutation in cancers and genetic diseases. Polε interacts with all three subunits of the PCNA ring to assemble a functional holoenzyme. Despite previous studies on proofreading of several Pol's, how Polε-or any Pol complexed with its sliding clamp-proofreads a mismatch generated in situ has been unknown. We show here by cryo-EM that a template/primer DNA substrate with a preexisting mismatch cannot enter the exo site of Polε-PCNA holoenzyme, but a mismatch generated in situ in the pol site yields three bona fide proofreading intermediates of Polε-PCNA holoenzyme. These intermediates reveal how the mismatch is dislodged from the pol site, how the DNA unwinds six base pairs, and how the unpaired primer 3'-end is inserted into the exo site for cleavage. These results unexpectedly demonstrate that PCNA imposes strong steric constraints that extend unwinding and direct the trajectory of mismatched DNA and that this trajectory is dramatically different than for Polε in the absence of PCNA. These findings suggest a physiologically relevant proofreading mechanism for the human Polε holoenzyme.
Keywords: DNA polymerase; DNA polymerase epsilon; DNA proofreading; leading strand; replication fidelity.
Conflict of interest statement
Competing interests statement:The authors declare no competing interest.
Figures



Update of
-
The proofreading mechanism of the human leading strand DNA polymerase ϵ holoenzyme.bioRxiv [Preprint]. 2025 Apr 12:2025.04.11.648458. doi: 10.1101/2025.04.11.648458. bioRxiv. 2025. Update in: Proc Natl Acad Sci U S A. 2025 Jun 3;122(22):e2507232122. doi: 10.1073/pnas.2507232122. PMID: 40291687 Free PMC article. Updated. Preprint.
Similar articles
-
The proofreading mechanism of the human leading strand DNA polymerase ϵ holoenzyme.bioRxiv [Preprint]. 2025 Apr 12:2025.04.11.648458. doi: 10.1101/2025.04.11.648458. bioRxiv. 2025. Update in: Proc Natl Acad Sci U S A. 2025 Jun 3;122(22):e2507232122. doi: 10.1073/pnas.2507232122. PMID: 40291687 Free PMC article. Updated. Preprint.
-
Structures of the human leading strand Polε-PCNA holoenzyme.Nat Commun. 2024 Sep 8;15(1):7847. doi: 10.1038/s41467-024-52257-x. Nat Commun. 2024. PMID: 39245668 Free PMC article.
-
Replication protein A dynamically re-organizes on primer/template junctions to permit DNA polymerase δ holoenzyme assembly and initiation of DNA synthesis.Nucleic Acids Res. 2024 Jul 22;52(13):7650-7664. doi: 10.1093/nar/gkae475. Nucleic Acids Res. 2024. PMID: 38842913 Free PMC article.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19.Cochrane Database Syst Rev. 2022 May 20;5(5):CD013665. doi: 10.1002/14651858.CD013665.pub3. Cochrane Database Syst Rev. 2022. PMID: 35593186 Free PMC article.
References
-
- Kornberg A., DNA replication. Trends Biochem. Sci. 9, 122–124 (1984).
-
- Langston L. D., O’Donnell M., DNA replication: Keep moving and don’t mind the gap. Mol. Cell. 23, 155–160 (2006). - PubMed
-
- Johnson A., O’Donnell M., Cellular DNA replicases: Components and dynamics at the replication fork. Annu. Rev. Biochem. 74, 283–315 (2005). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

