First-line serplulimab plus chemotherapy in extensive-stage small-cell lung cancer: Updated results and biomarker analysis from the ASTRUM-005 randomized clinical trial
- PMID: 40440184
- PMCID: PMC12365545
- DOI: 10.1002/cac2.70032
First-line serplulimab plus chemotherapy in extensive-stage small-cell lung cancer: Updated results and biomarker analysis from the ASTRUM-005 randomized clinical trial
Abstract
Background: The ASTRUM-005 study previously demonstrated a significant overall survival (OS) benefit with serplulimab (a programmed death 1 inhibitor) plus chemotherapy versus chemotherapy alone in previously untreated extensive-stage small-cell lung cancer (ES-SCLC). Here, we report updated efficacy and safety results after an extended median follow-up of 19.8 months, along with the first report on findings from exploratory biomarker analyses.
Methods: A total of 585 patients were randomized in a 2:1 ratio to receive 4.5 mg/kg serplulimab (n = 389) or placebo (n = 196) intravenously every 3 weeks, together with carboplatin and etoposide. The primary endpoint was OS. In addition, genomic profiling was performed to identify mutated genes, and quantitative serum proteome profiling was conducted to identify differentially expressed proteins (DEPs) between responders and non-responders of serplulimab plus chemotherapy. Regression analysis was subsequently used to construct a protein signature based on the DEPs. The associations between efficacy outcomes (objective response rate [ORR], OS, and progression-free survival [PFS]) and gene mutation status or DEP expression were also examined with regression analysis. Furthermore, the prognostic value of hematological parameters was evaluated.
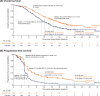
Results: In the intent-to-treat population, the median OS was 15.8 months in the serplulimab group versus 11.1 months in the placebo group (hazard ratio, 0.62; 95% confidence interval, 0.50-0.76; P < 0.001). We identified 181 DEPs between responders and non-responders in the serplulimab group, from which a 15-protein signature was constructed. In the serplulimab group, patients with a higher 15-protein signature score were associated with significantly longer OS and PFS. Also, patients harboring tumor-suppressor retinoblastoma-1 (RB1) mutations or mutations in Notch pathway members showed improved ORR, OS, or PFS compared with their wild-type counterparts. Baseline neutrophil-to-lymphocyte ratio (NLR) and lactate dehydrogenase (LDH) level were independent prognosticators of patients with ES-SCLC.
Conclusions: First-line serplulimab provided a sustained clinical benefit over placebo in patients with ES-SCLC. A 15-protein signature and mutations in RB1 or Notch pathway genes may serve as predictive biomarkers for benefits from serplulimab plus chemotherapy, while baseline NLR and LDH were independent prognosticators for ES-SCLC.
Keywords: ASTRUM‐005; ES‐SCLC; Serplulimab; phase 3.
© 2025 The Author(s). Cancer Communications published by John Wiley & Sons Australia, Ltd on behalf of Sun Yat‐sen University Cancer Center.
Conflict of interest statement
Haoyu Yu, Jing Li, Fang Yang, Xinyi Yang, Chen Ling, Qingyu Wang, Yongqiang Shan, and Jun Zhu have disclosed that they are employees of Shanghai Henlius Biotech, Inc. No other disclosures were reported.
Figures


References
-
- American Cancer Society . Cancer facts & figures. Published March 2021. Accessed October 28, 2022. https://www.cancer.org/content/dam/cancer‐org/research/cancer‐facts‐and‐...
-
- Zugazagoitia J, Paz‐Ares L. Extensive‐stage small‐cell lung cancer: first‐line and second‐line treatment options. J Clin Oncol. 2022;40(6):671–80. - PubMed
-
- Horn L, Mansfield AS, Szczęsna A, Havel L, Krzakowski M, Hochmair MJ, et al. First‐Line Atezolizumab plus Chemotherapy in Extensive‐Stage Small‐Cell Lung Cancer. N Engl J Med. 2018;379(23):2220–9. - PubMed
-
- Paz‐Ares L, Dvorkin M, Chen Y, Reinmuth N, Hotta K, Trukhin D, et al. Durvalumab plus platinum‐etoposide versus platinum‐etoposide in first‐line treatment of extensive‐stage small‐cell lung cancer (CASPIAN): a randomised, controlled, open‐label, phase 3 trial. Lancet. 2019;394(10212):1929–39. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

