Saliva urea nitrogen for detection of kidney disease in adults: A meta-analysis of diagnostic test accuracy
- PMID: 40440346
- PMCID: PMC12121763
- DOI: 10.1371/journal.pone.0324251
Saliva urea nitrogen for detection of kidney disease in adults: A meta-analysis of diagnostic test accuracy
Abstract
Background: Kidney disease affects millions globally, especially in low and middle-income countries where access to diagnostic testing is limited. Saliva urea nitrogen (SUN) has been proposed as a simple, non-invasive alternative to traditional serum-based diagnostics.
Objective: This study aimed to evaluate the diagnostic accuracy of SUN for detecting kidney disease in adults through a systematic review and meta-analysis.
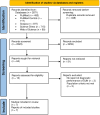
Methods: This review adhered to the PRISMA-DTA guidelines. A comprehensive search of five databases was conducted without language or date restrictions. Study quality was assessed using the QUADAS-2 tool. STATA version 17 was used for analysis. A random-effects model was used to estimate pooled sensitivity, specificity, and diagnostic odds ratios (DOR). Subgroup analysis was conducted based on the reference test used (serum creatinine or blood urea nitrogen). Heterogeneity was assessed using the I² statistic, and meta-regression explored sources of heterogeneity.
Results: Seven studies (n = 1,933) met the inclusion criteria. In the serum creatinine (sCr) subgroup (2 studies), SUN showed pooled sensitivity of 0.44 (95% CI: 0.38-0.49), specificity 0.96 (95% CI: 0.95-0.98), DOR 18.89 (95% CI: 15.19-23.57), and AUC ~ 0.90. In the blood urea nitrogen (BUN) subgroup (5 studies), sensitivity was 0.83 (95% CI: 0.69-0.91), specificity 0.88 (95% CI: 0.78-0.94), DOR 37 (95% CI: 15-91), and AUC 0.93. Heterogeneity was moderate in the BUN subgroup (bivariate I² = 51%), with 42% of variability attributed to threshold effects. Meta-regression identified study country (p = 0.01), and reference test used (p = 0.02) as contributors to heterogeneity in sensitivity, while comorbidity (p = 0.001) significantly affected specificity.
Conclusion: SUN shows high diagnostic specificity and a good overall accuracy, particularly when compared to BUN, and may serve as a practical non-invasive screening tool in low- resource settings. While heterogeneity was present, SUN remains a promising diagnostic alternative and warrants further validation in diverse clinical populations.
Copyright: © 2025 Kumaran et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures






Similar articles
-
Diagnostic performance of salivary urea nitrogen dipstick to detect and monitor acute kidney disease in patients with malaria.Malar J. 2018 Dec 18;17(1):477. doi: 10.1186/s12936-018-2627-4. Malar J. 2018. PMID: 30563520 Free PMC article.
-
Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.Cochrane Database Syst Rev. 2022 Feb 1;2(2022):CD014217. doi: 10.1002/14651858.CD014217. Cochrane Database Syst Rev. 2022. PMID: 36321557 Free PMC article.
-
Saliva urea nitrogen dipstick - a novel bedside diagnostic tool for acute kidney injury.Clin Nephrol. 2014 Dec;82(6):358-66. doi: 10.5414/CN108370. Clin Nephrol. 2014. PMID: 25345383
-
Saliva urea dipstick test: application in chronic kidney disease.Clin Nephrol. 2011 Jul;76(1):23-8. doi: 10.5414/cn106826. Clin Nephrol. 2011. PMID: 21722602
-
Saliva as an alternative to blood in the determination of uremic state in adult patients with chronic kidney disease: a systematic review and meta-analysis.Clin Oral Investig. 2020 Jul;24(7):2203-2217. doi: 10.1007/s00784-020-03340-2. Epub 2020 May 23. Clin Oral Investig. 2020. PMID: 32447524
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

