Conserved brain-wide emergence of emotional response from sensory experience in humans and mice
- PMID: 40440375
- PMCID: PMC12286656
- DOI: 10.1126/science.adt3971
Conserved brain-wide emergence of emotional response from sensory experience in humans and mice
Abstract
Emotional responses to sensory experience are central to the human condition in health and disease. We hypothesized that principles governing the emergence of emotion from sensation might be discoverable through their conservation across the mammalian lineage. We therefore designed a cross-species neural activity screen, applicable to humans and mice, combining precise affective behavioral measurements, clinical medication administration, and brain-wide intracranial electrophysiology. This screen revealed conserved biphasic dynamics in which emotionally salient sensory signals are swiftly broadcast throughout the brain and followed by a characteristic persistent activity pattern. Medication-based interventions that selectively blocked persistent dynamics while preserving fast broadcast selectively inhibited emotional responses in humans and mice. Mammalian emotion appears to emerge as a specifically distributed neural context, driven by persistent dynamics and shaped by a global intrinsic timescale.
Conflict of interest statement
Figures
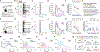


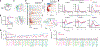


Comment in
-
A wave of emotion.Science. 2025 May 29;388(6750):917-918. doi: 10.1126/science.adx8992. Epub 2025 May 29. Science. 2025. PMID: 40440399
References
-
- Bradley MM, Lang PJ, in Cognitive Neuroscience of Emotion (Oxford Univ. Press, 1999), pp. 242–276.
-
- Darwin C, The Expression of the Emotions in Man and Animals (John Murray, 1872). doi: 10.1037/10001-000 - DOI
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

