Efficacy, Safety and Tolerability of Dispersible and Immediate Release Abacavir/Dolutegravir/Lamivudine Tablets in Children With HIV: IMPAACT 2019 Week 48 Results
- PMID: 40440679
- PMCID: PMC12221218
- DOI: 10.1097/INF.0000000000004859
Efficacy, Safety and Tolerability of Dispersible and Immediate Release Abacavir/Dolutegravir/Lamivudine Tablets in Children With HIV: IMPAACT 2019 Week 48 Results
Abstract
Background: Dispersible and immediate-release fixed-dose combinations (FDC) of abacavir, dolutegravir, and lamivudine are priority first-line antiretroviral therapy (ART) in children with HIV-1 (CWHIV). We report safety, efficacy and tolerability of these regimens through 48 weeks of treatment.
Methods: IMPAACT 2019 was a phase I/II, international, multisite, open-label, noncomparative study of dispersible and immediate-release FDC abacavir/dolutegravir/lamivudine (ABC/DTG/3TC) in participants with HIV-1 <12 years of age weighing 6 to <40 kg. At entry, participants were ART-naive or ART-experienced and virally suppressed on stable ART for ≥6 months. Participants received weight-banded dosing and enrolled across 5 weight bands in parallel. Follow-up visits were completed at weeks 1, 4, 12, 24, 36 and 48.
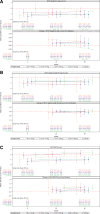
Results: Fifty-seven participants were enrolled; 2 participants withdrew due to poor drug tolerability. Fifty-four of 55 participants on the study at week 48 remained on the study drug. All 54 participants who remained on study drug through week 48 had viral loads of <200 copies/mL. CD4-lymphocyte counts remained stable with age over 48 weeks. Mean change (95% confidence interval) in body mass index Z-scores was 0.4 (0.2-0.6). Nine study drug-related adverse events were reported. One drug-induced liver injury attributed to abacavir and dolutegravir led to the permanent discontinuation of the study drug.
Conclusions: Dispersible FDC ABC/DTG/3TC is the first dispersible dolutegravir-containing single tablet regimen for CWHIV. Dispersible- and immediate-release ABC/DTG/3TC was observed to be generally safe, effective and well-tolerated in CWHIV through 48 weeks.
Trial registration: ClinicalTrials.gov NCT00376045.
Keywords: antiretroviral; fixed-dose combination; integrase inhibitor; nucleoside reverse transcriptase inhibitors; pediatric.
Copyright © 2025 The Author(s). Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
H.R. and K.M.B. have received consulting fees from ViiV Healthcare. L.Z., S.W. and Y.R. received National Institutes of Health funding and ViiV Healthcare funding, both paid to their institutions. D.E.Y. is an employee of the National Institutes of Health (National Institute of Allergy and Infectious Diseases), who sponsored the study, and was formerly an unpaid technical advisor for the nonprofits Cover the Globe and Maipelo Trust. H.C. holds stock in GlaxoSmithKline and is an employee of GlaxoSmithKline. A.M.B. and C.B. hold stock in GlaxoSmithKline and are employees of ViiV Healthcare. The other authors have no conflicts of interest to disclose.
Figures
References
-
- The urgency of now: AIDS at a crossroads. Joint United Nations Programme on HIV/AIDS; 2024. Licence: CC BY-NC-SA 3.0 IGO. Available at: https://www.unaids.org/en/resources/documents/2024/global-aids-update-2024. Accessed August 5, 2024.
-
- Penazzato M, Townsend CL, Sam-Agudu NA, et al. ; PADO-HIV 5 participants. Advancing the prevention and treatment of HIV in children: priorities for research and development. Lancet HIV. 2022;9:e658–e666. - PubMed
-
- Consolidated guidelines on HIV prevention, testing, treatment, service delivery and monitoring: recommendations for a public health approach. World Health Organization; 2021. Licence: CC BY-NC-SA 3.0 IGO. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

