Mouse liver assembloids model periportal architecture and biliary fibrosis
- PMID: 40441268
- PMCID: PMC12350178
- DOI: 10.1038/s41586-025-09183-9
Mouse liver assembloids model periportal architecture and biliary fibrosis
Abstract
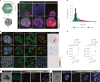
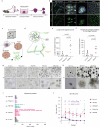
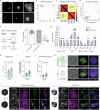
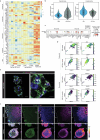
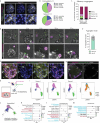
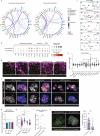
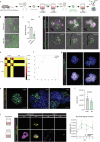
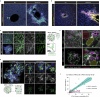
Modelling liver disease in vitro requires systems that replicate disease progression1,2. Current tissue-derived organoids do not reproduce the complex cellular composition and tissue architecture observed in vivo3. Here, we describe a multicellular organoid system composed of adult hepatocytes, cholangiocytes and mesenchymal cells that recapitulates the architecture of the liver periportal region and, when manipulated, models aspects of cholestatic injury and biliary fibrosis. We first generate reproducible hepatocyte organoids with a functional bile canaliculi network that retain morphological features of in vivo tissue. By combining these with cholangiocytes and portal fibroblasts, we generate assembloids that mimic the cellular interactions of the periportal region. Assembloids are functional, consistently draining bile from bile canaliculi into the bile duct. Of note, manipulating the relative number of portal mesenchymal cells is sufficient to induce a fibrotic-like state, independently of an immune compartment. By generating chimeric assembloids of mutant and wild-type cells, or after gene knockdown, we show proof of concept that our system is amenable to investigating gene function and cell-autonomous mechanisms. Together, we demonstrate that liver assembloids represent a suitable in vitro system to study bile canaliculi formation, bile drainage and how different cell types contribute to cholestatic disease and biliary fibrosis in an all-in-one model.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: M.H. is inventor in several patents on organoid technology. A.S., A.M.D. are inventors in a patent on organoids. The other authors declare no competing interests.
Figures











References
-
- Pinzani, M. & Luong, T. V. Pathogenesis of biliary fibrosis. Biochim. Biophys. Acta1864, 1279–1283 (2018). - PubMed
-
- Campana, L., Esser, H., Huch, M. & Forbes, S. Liver regeneration and inflammation: from fundamental science to clinical applications. Nat. Rev. Mol. Cell Biol.22, 608–624 (2021). - PubMed
-
- Fausto, N., Campbell, J. S. & Riehle, K. J. Liver regeneration. J. Hepatol.57, 692–694 (2012). - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources

