Light-Driven Enzyme Catalysis: Ultrafast Mechanisms and Biochemical Implications
- PMID: 40441704
- PMCID: PMC12177928
- DOI: 10.1021/acs.biochem.5c00039
Light-Driven Enzyme Catalysis: Ultrafast Mechanisms and Biochemical Implications
Abstract
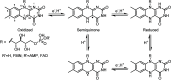
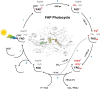
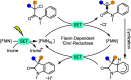
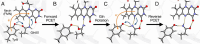
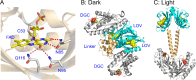
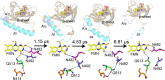
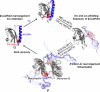
Light-activated enzymes are an important class of biocatalysts in which light energy is directly converted into biochemical activity. In most cases the light absorbing group is the isoalloxazine ring of an embedded flavin cofactor and in general two types of mechanism are in operation depending on whether the excited chromophore directly participates in catalysis or where photoexcitation triggers conformational changes that modulate the activity of a downstream output partner. This review will summarize studies on DNA photolyase, fatty acid photodecarboxylase (FAP), the monooxygenase PqsL, and flavin-dependent ene-reductases, where flavin radicals generated by excitation are directly used in the reactions catalyzed by these enzymes, and the blue light using FAD (BLUF) and light oxygen voltage (LOV) domain photoreceptors where flavin excitation drives ultrafast structural changes that ultimately result in enzyme activation. Recent advances in methods such as time-resolved spectroscopy and structural imaging have enabled unprecedented insight into the ultrafast dynamics that underly the mechanism of light-activated enzymes, and here we highlight how understanding ultrafast protein dynamics not only provides valuable insights into natural phototransduction processes but also opens new avenues for enzyme engineering and consequent applications in fields such as optogenetics.
Copyright © 2025 The Authors. Published by American Chemical Society
Conflict of interest statement
The authors declare no competing financial interest.
Figures




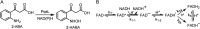




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

