Drug treatments for mild or moderate covid-19: systematic review and network meta-analysis
- PMID: 40441732
- PMCID: PMC12120598
- DOI: 10.1136/bmj-2024-081165
Drug treatments for mild or moderate covid-19: systematic review and network meta-analysis
Abstract
Objective: To compare the effects of treatments for mild or moderate (that is, non-severe) coronavirus disease 2019 (covid-19).
Design: Systematic review and network meta-analysis.
Data sources: Covid-19 Living Overview of Evidence Repository (covid-19 L-OVE) by the Epistemonikos Foundation, a public, living repository of covid-19 articles, from 1 January 2023 to 19 May 2024. The search also included the WHO covid-19 database (up to 17 February 2023) and six Chinese databases (up to 20 February 2021). The analysis included studies identified between 1 December 2019 and 28 June 2023.
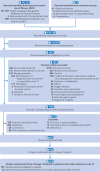
Study selection: Randomised clinical trials in which people with suspected, probable, or confirmed mild or moderate covid-19 were allocated to drug treatment or to standard care or placebo. Pairs of reviewers independently screened potentially eligible articles.
Methods: After duplicate data abstraction, a bayesian network meta-analysis was conducted. Risk of bias was assessed by use of a modification of the Cochrane risk of bias 2.0 tool, and the certainty of the evidence using the grading of recommendations assessment, development, and evaluation (GRADE) approach. For each outcome, following GRADE guidance, drug treatments were classified in groups from the most to the least beneficial or harmful.
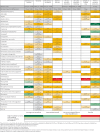
Results: Of 259 trials enrolling 166 230 patients, 187 (72%) were included in the analysis. Compared with standard care, two drugs probably reduce hospital admission: nirmatrelvir-ritonavir (25 fewer per 1000 (95% confidence interval 28 fewer to 20 fewer), moderate certainty) and remdesivir (21 fewer per 1000 (28 fewer to 7 fewer), moderate certainty). Molnupiravir and systemic corticosteroids may reduce hospital admission (low certainty). Compared with standard care, azithromycin probably reduces time to symptom resolution (mean difference 4 days fewer (5 fewer to 3 fewer), moderate certainty) and systemic corticosteroids, favipiravir, molnupiravir, and umifenovir probably also reduce duration of symptoms (moderate to high certainty). Compared with standard care, only lopinavir-ritonavir increased adverse effects leading to discontinuation.
Conclusion: Nirmatrelvir-ritonavir and remdesivir probably reduce admission to hospital, and systemic corticosteroids and molnupiravir may reduce admission to hospital. Several medications including systemic corticosteroids and molnupiravir probably reduce time to symptom resolution.
Systematic review registration: This review was not registered. The protocol is publicly available in the supplementary material.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/disclosure-of-interest/ and declare: support from the Canadian Institutes of Health Research for the submitted work; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
Figures
References
-
- World Health Organization. WHO COVID-19 dashboard. 2023. https://covid19.who.int.
-
- COVID-19 Studies from the World Health Organization Database - ClinicalTrials.gov. 2023 https://classic.clinicaltrials.gov/ct2/who_table.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources