Development of bivalent RBD adapted COVID-19 vaccines for broad sarbecovirus immunity
- PMID: 40442110
- PMCID: PMC12122808
- DOI: 10.1038/s41541-025-01156-3
Development of bivalent RBD adapted COVID-19 vaccines for broad sarbecovirus immunity
Abstract
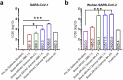
COVID-19 vaccine adaptation is critical to respond to continuously emerging SARS-CoV-2 variants with enhanced immune evasion. The ARVAC protein subunit vaccine, based on the receptor binding domain of the spike protein of SARS-CoV-2, has been adapted to XBB.1.5 and JN.1 variants, as monovalent and bivalent formulations. Preclinical studies in mice showed that ARVAC XBB.1.5 and JN.1 monovalent vaccines induced strong neutralizing antibodies against XBB and JN.1 lineages, though with limited efficacy against phylogenetically distant variants. By contrast, bivalent formulations combining Gamma antigen with either XBB.1.5 or JN.1 antigens demonstrated superior cross-neutralizing activity, covering variants from Ancestral to JN.1. Additionally, Gamma-containing bivalent vaccines elicited neutralizing antibodies against SARS-CoV-1, highlighting their potential for broad-spectrum immunity. Cellular immune studies confirmed robust CD4+ T cell activation across all formulations. These findings support the continued adaptation of ARVAC to current circulant variants and propose ARVAC bivalent vaccines containing the Gamma antigen as a strategy for induction of pan-sarbecovirus immunity.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: J.M.R. and A.C.H.I. are salaried employees of Fundacion Pablo Cassara. J.M.F., S.A.D.P., I.G.K. and J.C.V. are salaried employees of Laboratorio Pablo Cassara. L.M.C., L.A.B., C.P.C., A.D., L.P., C.G.F.C., L.M.S., F.P.C., J.C. and K.A.P. declare no competing interests relevant to this article. G.P., R.A. and G.L. are employees of VisMederi srl. N.T. declares no competing interests.
Figures




References
-
- Bennett, C. et al. Immunogenicity and safety of a bivalent (Omicron BA.5 plus ancestral) SARS-CoV-2 recombinant spike protein vaccine as a heterologous booster dose: interim analysis of a phase 3, non-inferiority, randomised, clinical trial. Lancet Infect. Dis.24, 581–593 (2024). - PubMed
-
- WHO. Statement on the antigen composition of COVID-19 vaccines. https://www.who.int/news/item/26-04-2024-statement-on-the-antigen-compos... (2023).
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

