Serum proteomic approach to identifying differentially expressed proteins in effusive feline infectious peritonitis
- PMID: 40442209
- PMCID: PMC12122842
- DOI: 10.1038/s41598-025-03108-2
Serum proteomic approach to identifying differentially expressed proteins in effusive feline infectious peritonitis
Abstract
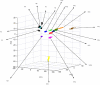
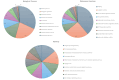
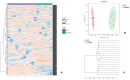
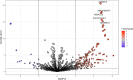
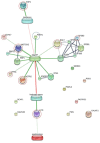
Feline infectious peritonitis (FIP) is a lethal, viral-induced immune-mediated disease that remains a challenge for diagnosis and treatment in cats. Proteomic profiling, which analyzes the protein content of biological samples, offers the potential to identify novel biomarkers that could improve the diagnosis and management of FIP. This study aims to assess the serum proteome and identify proteins that differentiate healthy cats from cats diagnosed with effusive FIP using liquid chromatography coupled with tandem mass spectrometry (LC-MS/MS). A total of 30 cats diagnosed with effusive FIP and 27 clinically normal cats were enrolled. Twenty-three proteins were significantly (p < 0.01, ≥ fivefold change in abundance) differentially expressed between cats with effusive FIP and controls. Among these, the P2X purinoceptor, DNA topoisomerase, Notch receptor 2, and cadherin-17 were identified as key proteins of interest in cats with effusive FIP. Our findings suggest that these differentially expressed proteins could serve as potential diagnostic biomarkers and therapeutic targets for FIP. However, further studies are needed to validate these findings and explore their potential applications.
Keywords: Biomarkers; Cats; Coronavirus; Feline infectious peritonitis; Proteomics; Serum.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests. Ethics approval and consent participate: The work described in this manuscript involved the use of non-experimental (owned) animals. The study received prior ethical approval from the Kasetsart University Institutional Animal Care and Use Committee, Kasetsart University, Bangkok, Thailand, which approved all procedures involving animal use (approval number: ACKU66-VET-019). This study is reported in accordance with ARRIVE guidelines. All methods were conducted in accordance with relevant guidelines and regulations for reporting animal experiments. Blood for proteomic analysis was collected during routine veterinary examinations. Serum samples were obtained from the patients with signed informed consent forms from their owners, and a high standard of care was maintained throughout each examination.
Figures


References
-
- Holzworth, J. Some important disorders of cats. Cornell Vet.53, 157–160 (1963). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

