Aphid Colony Size in Tansy is Affected by Plant Chemical Composition but not by Belowground Herbivory
- PMID: 40442448
- PMCID: PMC12122609
- DOI: 10.1007/s10886-025-01609-y
Aphid Colony Size in Tansy is Affected by Plant Chemical Composition but not by Belowground Herbivory
Abstract
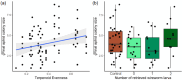
Plants are hosts for above- and belowground insect communities that can influence each other via above-belowground plant-physiological dynamics. To mediate interactions, plants produce secondary metabolites, including terpenoids, and mixtures can differ intraspecifically. While intraspecific variation in plant chemistry gained increased interest, the extent to which intraspecific differences in plant chemistry mediate above-belowground interactions of herbivores remains unclear. We used a full factorial design with six distinct terpenoid chemotypes, differing in their chemical diversity of tansy (Tanacetum vulgare). We exposed these to the aboveground herbivore Macrosiphoniella tanacetaria (Hemiptera: Aphididae), the belowground herbivore Agriotes sp. (Coleoptera: Elateridae), no herbivore or both herbivores, to determine if chemotypes or the chemical diversity of plant compounds affected aphid performance and if the interactions between herbivores were mediated by the chemical profile. We found that aphid colony size differed between chemotypes, with the strongest colony increase over time in a mixed chemotype, and the weakest in a β-thujone chemotype. Root herbivory had no effect on aphid colony size, regardless of the chemotype. Aphid colony size was positively correlated with terpenoid evenness, but not with terpenoid Shannon diversity, terpenoid richness, or relative terpenoid concentration. Tansy chemotypes differed in their morphological responses (final plant height and final plant dry weight) and average leaf chlorophyll content to aboveground herbivory, whereas belowground herbivory exerted minimal impacts. Overall, our results show that intraspecific variation in terpenoid profiles directly modify ecological interactions on a plant, with plant chemistry mediating aphid performance and chemotypes differing in their morphological responses to herbivory.
Keywords: Tanacetum vulgare; Herbivory; Intraspecific chemodiversity; Plant–insect interactions; Terpenoids.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interest: The authors declare no competing interests.
Figures



References
-
- Adams RP (2017) Identification of essential oil components by gas chromatography/mass spectrometry (5, online. Texensis Publishing, Gruver, TX
-
- Bączek K, Pióro-Jabrucka E, Kosakowska O, Węglarz Z (2019) Intraspecific variability of wild thyme (Thymus serpyllum L.) occurring in Poland. J Appl Res Med Aromatic Plants 12:30–35. 10.1016/j.jarmap.2018.11.001 - DOI
-
- Bates D, Mächler M, Bolker B, Walker S (2014) Fitting Linear Mixed-Effects Models using lme4 (arXiv:1406.5823). arXiv. 10.48550/arXiv.1406.5823
-
- Benedek K, Bálint J, Salamon RV, Kovács E, Ábrahám B, Fazakas C, Loxdale HD, Balog A (2015) Chemotype of tansy (Tanacetum vulgare L.) determines aphid genotype and its associated predator system: plant chemotype and aphid genotype-predator interaction. Biol J Linnean Soc 114(3):709–719. 10.1111/bij.12445 - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

