Safety and efficacy of sirolimus combined with cyclosporine in primary membranous nephropathy: a randomized controlled trial
- PMID: 40442735
- PMCID: PMC12123714
- DOI: 10.1186/s12916-025-04173-0
Safety and efficacy of sirolimus combined with cyclosporine in primary membranous nephropathy: a randomized controlled trial
Abstract
Background: Calcineurin inhibitors, such as cyclosporine, are primary treatments for membranous nephropathy (MN). Optimizing this regimen is crucial to reduce nephrotoxicity and enhance immunological remission. Sirolimus, when combined with cyclosporine, may offer non-inferior clinical remission to cyclosporine monotherapy, while improving kidney function preservation and antibody clearance.
Methods: This single-center, randomized controlled, phase 2 clinical trial involved 74 patients with biopsy-proven primary MN and persistent proteinuria > 3.5 g/d, despite 6 months of supportive care. Participants were randomly assigned (1:1) to receive either sirolimus plus cyclosporine or cyclosporine monotherapy for 12 months.
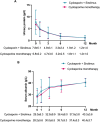
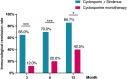
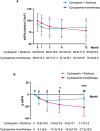
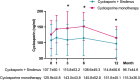
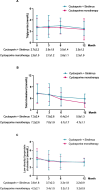
Results: At 12 months, composite remission was achieved in 26 of 36 patients (72%) receiving combination therapy and in 24 of 36 (67%) receiving cyclosporine alone (95% confidence interval for noninferiority: 0.48-3.56). Immunological remission, defined as anti-phospholipase A2 receptor antibody conversion from positive to negative, was significantly higher in the combination group (70% vs. 20%, P < 0.001) at 6 months. Moreover, eGFR decline was significantly attenuated in the combination group (ΔeGFR: -7.1 vs. -21.3 ml/min/1.73m2, P < 0.001) at 12 months. One serious adverse event occurred in the combination group, compared to none in the monotherapy group (P = 0.317).
Conclusions: Sirolimus combined with cyclosporine was noninferior to cyclosporine monotherapy in achieving clinical remission in MN and demonstrated superior benefits in immunological remission and kidney function preservation.
Keywords: Anti-PLA2R Antibody; Clinical Trial; Cyclosporine; Membranous Nephropathy; Sirolimus.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The trial protocol was designed by the principal investigators and approved by an independent ethics committee (approval number: 2017[1346]). The study was conducted in accordance with the Declaration of Helsinki, and all participants provided written informed consent prior to enrollment. The trial was registered with the Chinese Clinical Trial Registry (ChiCTR-INR-17012212). Consent for publication: Not applicable. This manuscript does not contain any individual person’s data in any form (including images, videos, or case details) that would require consent for publication. Competing interests: The authors declare no competing interests.
Figures
References
-
- Hoxha E, Reinhard L, Stahl RAK. Membranous nephropathy: new pathogenic mechanisms and their clinical implications. Nat Rev Nephrol. 2022;18(7):466–78. - PubMed
-
- Troyanov S, Wall CA, Miller JA, Scholey JW, Cattran DC. Idiopathic membranous nephropathy: definition and relevance of a partial remission. Kidney Int. 2004;66:1199–205. - PubMed
-
- Kidney Disease: Improving Global Outcomes (KDIGO) Glomerulonephritis Work Group. KDIGO clinical practice guideline for glomerulonephritis. Kidney Int. 2021; 100: S1–276.
-
- Ponticelli C, Zucchelli P, Imbasciati E, et al. Controlled trial of methylprednisolone and chlorambucil in idiopathic membranous nephropathy. N Engl J Med. 1984;310:946–50. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

