GPR56/ADGRG1 induces biased Rho-ROCK-MLC and JAK-STAT3 signaling to promote amoeboid-like morphology and IL-6 upregulation in melanoma cells
- PMID: 40442782
- PMCID: PMC12123722
- DOI: 10.1186/s12964-025-02267-z
GPR56/ADGRG1 induces biased Rho-ROCK-MLC and JAK-STAT3 signaling to promote amoeboid-like morphology and IL-6 upregulation in melanoma cells
Abstract
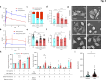
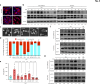
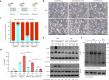
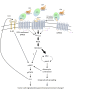
Background: GPR56/ADGRG1 is an adhesion G protein-coupled receptor involved in cell-matrix interactions and metastasis of human melanoma cells. Previously, we demonstrated that GPR56 activation in melanoma cells triggers Gα12/13-RhoA signaling, leading to increased IL-6 production and enhanced cell migration. Yet little is known of the downstream signaling effectors and their specific roles in regulating melanoma cellular phenotypes.
Results: In this study, we show that GPR56 activation induces Rho-ROCK-MLC and JAK-STAT3 signaling, which temporally and differentially drive amoeboid-like morphology and IL-6 upregulation. Interestingly, GPR56-induced JAK-STAT3 activation is partially regulated by Rho-ROCK-MLC signaling but not vice versa. Moreover, receptor auto-proteolysis modulates the magnitude of GPR56-mediated signaling, and its unique intracellular regions contribute to the selective regulation of unique signaling pathways and associated cellular phenotypes.
Conclusion: Our findings reveal complex GPR56-mediated biased signaling through the Rho-ROCK-MLC and JAK-STAT3 pathways, highlighting these networks as potential therapeutic targets for modulating distinct tumorigenic phenotypes in human melanoma cells.
Keywords: Adhesion GPCR; Cytoskeletal remodelling; GPR56; IL-6; Melanoma; Signalling.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests. Competing financial interests: The authors declare no competing financial interests.
Figures




References
MeSH terms
Substances
Grants and funding
- NSTC-108-2811-B-182-507, NSTC-110-2320-B-182-024, NSTC-113-2918-I-182-001, NSTC-113-2320-B-182-009/National Science and Technology Council
- NSTC-108-2811-B-182-507, NSTC-110-2320-B-182-024, NSTC-113-2918-I-182-001, NSTC-113-2320-B-182-009/National Science and Technology Council
- CMRPG3M0461, CMRPG3M1941, CMRPD1M0033, CMRPD1M0323/Chang Gung Memorial Hospital, Linkou
- CMRPG3M0461, CMRPG3M1941, CMRPD1M0033, CMRPD1M0323/Chang Gung Memorial Hospital, Linkou
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

