A cohort of GFPT1 related congenital myasthenic syndrome in China: high frequency of c.331 c > t variant
- PMID: 40442802
- PMCID: PMC12124097
- DOI: 10.1186/s13023-025-03823-z
A cohort of GFPT1 related congenital myasthenic syndrome in China: high frequency of c.331 c > t variant
Abstract
Background: Glutamine-fructose-6-phosphate transaminase 1 (GFPT1) is the key enzyme initiating protein O- and N-glycosylation at the postsynaptic membrane. Variants in GFPT1 gene cause congenital myasthenic (GFPT1-CMS). However, the understanding of the phenotype and genetic spectrum of GFPT1-CMS remains limited.
Methods: A total of 24 patients with GFPT1-CMS from 22 Han Chinese families across four neuromuscular disease centers were included in this study. Clinical assessments involved detailed medical histories, muscle biopsies, and electrophysiological studies. GFPT1 variants were identified using targeted next-generation sequencing or WES. Additionally, published GFPT1-CMS case data from 2011 to 2024 were compiled and combined with this cohort for genotype-phenotype correlation analysis.
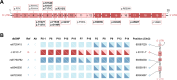
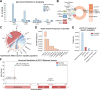
Results: In addition to the limb girdle myasthenia pattern, our cohort presented with extraocular involvement including eyelid ptosis and mild ophthalmoparesis (25.0%), facial weakness (20.8%) and a relatively high prevalence of distal weakness (62.5%). Electrophysiological testing revealed myopathic changes in 95.0% of cases and decremental CMAPs in all cases during RNS. We found that c.331 C > T is a hotspot variant in GFPT1-CMS patients and may have a founder effect in the Chinese population. Patients with homozygous null variants presented a more severe phenotype, including earlier onset and more frequent bulbar involvement.
Conclusion: We have described the clinical features and variant spectrum in a cohort of 24 Chinese GFPT1-CMS patients. Our findings update the understanding of clinical manifestation, pathological features and mutational spectrum in GFPT1-CMS patients.
Keywords: Congenital myasthenic syndrome; Distal muscle weakness; Glutamine-fructose-6-phosphate transaminase 1; Tubular aggregates.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval: This study was approved by the Institutional Review Board at Huashan Hospital, Fudan University (KY2021-753). Informed consent: Written informed consent was obtained from all participants. Competing interests: All authors declared no conflict of interest.
Figures

Similar articles
-
Congenital myasthenic syndrome with tubular aggregates caused by GFPT1 mutations.J Neurol. 2012 May;259(5):838-50. doi: 10.1007/s00415-011-6262-z. Epub 2011 Oct 6. J Neurol. 2012. PMID: 21975507
-
Novel compound heterozygous variants in the GFPT1 gene leading to rare limb-girdle congenital myasthenic syndrome with rimmed vacuoles.Neurol Sci. 2021 Aug;42(8):3485-3490. doi: 10.1007/s10072-020-05021-0. Epub 2021 Jan 13. Neurol Sci. 2021. PMID: 33438142
-
Global N-linked Glycosylation is Not Significantly Impaired in Myoblasts in Congenital Myasthenic Syndromes Caused by Defective Glutamine-Fructose-6-Phosphate Transaminase 1 (GFPT1).Biomolecules. 2015 Oct 16;5(4):2758-81. doi: 10.3390/biom5042758. Biomolecules. 2015. PMID: 26501342 Free PMC article.
-
Limb-girdle myasthenia with tubular aggregates associated with novel GFPT1 mutations.Muscle Nerve. 2012 Oct;46(4):600-4. doi: 10.1002/mus.23451. Muscle Nerve. 2012. PMID: 22987706 Review.
-
[Congenital myasthenic syndrome related to SLC25A1 gene variant: two cases report and literature review].Zhonghua Er Ke Za Zhi. 2021 Jan 2;59(1):42-46. doi: 10.3760/cma.j.cn112140-20200730-00767. Zhonghua Er Ke Za Zhi. 2021. PMID: 33397003 Review. Chinese.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

