IFN-α and vitamin B6-induced galectin-9 promotes the immunomodulatory function of human clonal mesenchymal stem cells
- PMID: 40442825
- PMCID: PMC12124064
- DOI: 10.1186/s13287-025-04394-3
IFN-α and vitamin B6-induced galectin-9 promotes the immunomodulatory function of human clonal mesenchymal stem cells
Abstract
Background: Mesenchymal stem cells (MSCs) possess a variety of immunomodulatory functions that can vary depending on the MSC line. Investigating priming strategies is essential for increasing the immunomodulatory potential of MSCs.
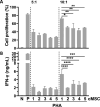
Methods: Human clonal MSCs (cMSCs) were primed with TNF-α, IFN-γ, IL-1β, IFN-α, and vitamin B6. Their immunomodulatory functions, including T-cell proliferation and cytokine production, were analyzed. The primed cMSCs were injected intravenously into a mouse model of ovalbumin-induced atopic dermatitis (AD), and their therapeutic effects were evaluated.
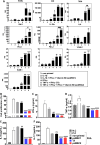
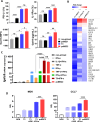
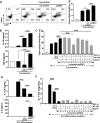
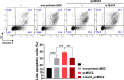
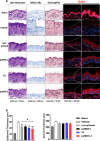
Results: We identified IFN-α and vitamin B6 as promising priming agents when they are combined with TNF-α and IFN-γ. The primed cMSCs showed expression of galectin-9 (Gal-9), IL-1Ra, and PDL-1. Gal-9 facilitates the induction of regulatory T cells (Tregs) and apoptosis. Treatment with primed cMSCs significantly alleviated pathological changes in an AD mouse model. Notable improvements included a reduction in epidermal thickness (p < 0.05), a decreased number of mast cells and eosinophils in the dermis (p < 0.01), restored expression of claudin-1 in the epidermis (p < 0.0001), and lower serum levels of IgE (p < 0.05).
Conclusions: This novel combination of priming factors significantly promotes the immunomodulatory functions of cMSCs by inducing Gal-9. Consequently, Gal-9 may serve as an excellent biomarker for screening primed cMSCs for their immunomodulatory capabilities, facilitating a more accurate assessment of their therapeutic effectiveness.
Keywords: Atopic dermatitis; Clonal mesenchymal stem cell; Galectin-9; IFN-α; Priming; Vitamin B6.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: This study includes samples from both humans and animals. The isolation of human cMSC from bone marrow samples was approved by three institutions: 1. The study titled “Bone Marrow Donation for Harvesting, Clinical Trial Researching, and Culturing of Stem Cell Therapeutics Bone Marrow-Derived Adult Stem Cells from Healthy Donors” received approval from the IRB of the Catholic University of Korea, Seoul St. Mary’s Hospital, with the approval number IRB No. KC15CSSE0336, dated December 14, 2014. 2. The study titled “Bone Marrow Donation for Harvesting and Culturing Bone Marrow-Derived Adult Stem Cells from Healthy Donors” was approved by the IRB of Inha University Hospital, with the approval number IRB No. 10–51, dated October 11, 2010. 3. The study titled “Clinical Grade Bone Marrow Collection for Clinical Research and/or Future Manufacturing of Human Cell Therapy Products” was approved by AllCells (Alameda, CA, USA), with the approval number IRB No. 949–542-3882, dated December 14, 2020. The use of PBMC from healthy donors has been approved. The study titled “Blood Donation from Healthy Donors for Analyzing the Immune Mechanism of Adult Stem Cells” received approval from the IRB of Inha University Hospital, with approval number IRB No. 2014–01-082–019, dated November 14, 2022. Written informed consent was obtained from all study participants. The “Efficacy Evaluation of Cell Therapy for the Treatment of Atopic Dermatitis” study received approval from the IACUC of Gachon University for the mouse experiments. The approval number is IACUC No. LCDI-2023–0054, dated June 14, 2023. Consent for publication: All authors gave consent for publication. Competing interests: The authors have declared no competing interests.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

