Evolution of the Military Health System compounded drugs utilization and management
- PMID: 40443000
- PMCID: PMC12123196
- DOI: 10.18553/jmcp.2025.31.6.537
Evolution of the Military Health System compounded drugs utilization and management
Abstract
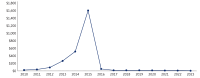
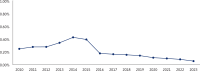
Over the past decade, the TRICARE pharmacy benefit has undergone significant changes, particularly in the management and utilization of compound drugs. Compound drugs are customized formulations that combine 2 or more pharmaceutical ingredients to meet specific patient needs and can offer therapeutic alternatives when standard US Food and Drug Administration-approved medications are ineffective. However, concerns regarding the safety, clinical effectiveness, and rising costs have necessitated increased oversight. Between 2013 and 2015, TRICARE experienced a drastic surge in compound drug expenditures, escalating from 4% of total pharmacy outpatient drug costs to 13% (more than $1.6 billion), despite representing only 0.4% of total outpatient prescription volume. This rapid increase highlighted the need for stricter controls to manage spending and ensure appropriate utilization. In response, the Defense Health Agency implemented a compound drug screening process in 2015, applying utilization management tools such as quantity limits, prior authorization, and step therapy. These measures aim to balance cost containment with maintaining access to clinically necessary compounded medications. This article provides a comprehensive review of the evolution of compound drugs within the TRICARE pharmacy benefit, examining safety concerns, spending trends, and management strategies.
Figures
Similar articles
-
Evolution of the TRICARE Pharmacy Benefit: A Decade of Change.J Manag Care Spec Pharm. 2019 Nov;25(11):1195-1200. doi: 10.18553/jmcp.2019.25.11.1195. J Manag Care Spec Pharm. 2019. PMID: 31663455 Free PMC article.
-
Utilization and Costs of Compounded Medications for Commercially Insured Patients, 2012-2013.J Manag Care Spec Pharm. 2016 Feb;22(2):172-81. doi: 10.18553/jmcp.2016.22.2.172. J Manag Care Spec Pharm. 2016. PMID: 27015256 Free PMC article.
-
Formulary management in the Department of Defense.J Manag Care Pharm. 2009 Mar;15(2):133-46. doi: 10.18553/jmcp.2009.15.2.133. J Manag Care Pharm. 2009. PMID: 19236127 Free PMC article.
-
Potential risks of pharmacy compounding.Drugs R D. 2013 Mar;13(1):1-8. doi: 10.1007/s40268-013-0005-9. Drugs R D. 2013. PMID: 23526368 Free PMC article. Review.
-
Prescription drug cost sharing: associations with medication and medical utilization and spending and health.JAMA. 2007 Jul 4;298(1):61-9. doi: 10.1001/jama.298.1.61. JAMA. 2007. PMID: 17609491 Free PMC article. Review.
References
-
- Defense Health Agency . Evaluation of the TRICARE Program: Fiscal Year 2023 Report to Congress. Access, cost, and quality data through fiscal year 2022. February 28, 2023. Accessed January 18, 2024. https://www.health.mil/Reference-Center/Reports/2023/09/07/Annual-Evalua...
-
- Sweeney E. After explosion of compounded drug fraud, legal experts say the party’s over. FierceHealthcare. February 24, 2015. Accessed February 12, 2024. https://www.fiercehealthcare.com/antifraud/fierce-exclusive-after-explos...
-
- Food and Drug Administration . Development & Approval Process - Drugs. Accessed April 9, 2024. https://www.fda.gov/drugs/development-approval-process-drugs
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous