Reductions in medical visits and hospitalizations following berotralstat initiation in patients with hereditary angioedema in the United States
- PMID: 40443005
- PMCID: PMC12123198
- DOI: 10.18553/jmcp.2025.31.6.578
Reductions in medical visits and hospitalizations following berotralstat initiation in patients with hereditary angioedema in the United States
Abstract
Background: Hereditary angioedema (HAE) is a rare disease characterized by unpredictable recurrent, debilitating, and potentially fatal attacks of subcutaneous and submucosal tissue swelling.
Objective: To evaluate all-cause, angioedema-related, and HAE attack-related medical visits and hospitalizations before and after initiation of berotralstat long-term prophylaxis (LTP) for patients with HAE in the United States.
Methods: This retrospective pre-post analysis used Komodo's Healthcare Map claims data to identify patients who initiated berotralstat (December 2020 to December 2022). The first entry for berotralstat dispensing was defined as the index date. Inclusion criteria comprised patients aged at least 12 years at index with at least 6 months of continuous insurance eligibility pre-index and evidence consistent with HAE pre-index (International Classification of Diseases, Tenth Revision, Clinical Modification diagnosis codes D84.1, D68.2, or T78.3x; medication use [on-demand or LTP]; or presence of diagnostic HAE laboratory tests). Rates of all-cause, angioedema-related, and HAE attack-related medical visits per person-year were compared post-index vs pre-index using rate ratios with 95% CIs and P values from generalized estimating equation Poisson regression models with robust SEs. Study limitations included the inability to distinguish HAE types and the uncertainty of whether a dispensed medication was consumed or taken as prescribed.
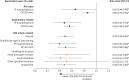
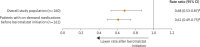
Results: The study population included 260 patients treated with berotralstat (mean age = 39.7 years; 74.2% female). After berotralstat initiation, there were significant decreases in the rates of all-cause health care resource utilization (HRU): all-cause inpatient (IP) visits decreased by 34% (P = 0.037) and all-cause outpatient/emergency department (OP/ED) visits decreased by 14% (P = 0.005). There were also significant decreases in rates of angioedema-related HRU (IP visits: 52%, P = 0.001; OP/ED visits: 44%, P < 0.001) as well as HAE attack-related HRU (IP visits: 60%, P < 0.001; OP/ED visits: 50%, P < 0.001). Use of on-demand medications decreased significantly after berotralstat initiation (32%, P = 0.002). Results were similar among subgroups of patients defined by HAE treatment history, including patients who were LTP-experienced (n = 126) and LTP-naive but on-demand treatment-experienced (n = 67).
Conclusions: Prophylactic treatment of HAE with berotralstat was associated with significant reductions in all-cause HRU, including decreases to angioedema-related and HAE attack-related medical visits, hospitalizations, and administration of on-demand treatment.
Conflict of interest statement
Dr Christiansen reports receiving consulting fees from BioCryst, Kalvista, and CSL and participating in the Medical Advisory Board for the US HAE Association. Dr Johnston is an employee of and owns stock/stock options in BioCryst Pharmaceuticals and reports receiving payment or honoraria from Takeda and BioCryst (before employment) and participating as co-Chair of the AAAAI Urticaria and angioedema subcommittee. Dr Lopez-Gonzalez is an employee of and owns stock/stock options in BioCryst Pharmaceuticals and reports participating as an unpaid volunteer for the National Adrenal Diseases Foundation Support Group Leader, Central Texas Adrenal Insufficiency Support Group. Drs Gillard and Nestler-Parr are employees of and own stock/stock options in BioCryst Pharmaceuticals. Mr MacKnight, Mr Laliberté, Ms Spencer, and Mr Boudreau are employees of Groupe d’Analyze, Ltée, which received funding from BioCryst Pharmaceuticals for this study. Dr Zuraw reports receiving grants or contracts from the Veterans Affairs Administration (Merit Grant) and Ionis (laboratory service agreement); royalties or licenses from UpToDate (royalties on chapters) and University of California (royalties on patent); consulting fees from BioCryst, CSL Behring, and Takeda; support for attending meetings and/or travel from the US HAE Association (travel to attend HAE Association meetings), CSL Behring (travel to Immunology Academy), and BioCryst (travel to speak); patents from the University of California (TSKA assay); participating as Chair of the Data Safety Monitoring Board for CSL Behring and BioMarin; and participating as Chair of the Medical Advisory Board for the US HAE Association. Drs Christiansen and Zuraw contributed equally.
Figures
Similar articles
-
Comparison of real-world healthcare resource utilization and costs among patients with hereditary angioedema on lanadelumab or berotralstat long-term prophylaxis.J Comp Eff Res. 2025 Apr;14(4):e240205. doi: 10.57264/cer-2024-0205. Epub 2025 Feb 20. J Comp Eff Res. 2025. PMID: 39976166 Free PMC article.
-
A Retrospective Analysis of Long-Term Prophylaxis with Berotralstat in Patients with Hereditary Angioedema and Acquired C1-Inhibitor Deficiency-Real-World Data.Clin Rev Allergy Immunol. 2023 Dec;65(3):354-364. doi: 10.1007/s12016-023-08972-2. Epub 2023 Nov 2. Clin Rev Allergy Immunol. 2023. PMID: 37914894 Free PMC article. Review.
-
Berotralstat (BCX7353) is a novel oral prophylactic treatment for hereditary angioedema: Review of phase II and III studies.Allergy Asthma Proc. 2021 Jul 14;42(4):274-282. doi: 10.2500/aap.2021.42.210034. Epub 2021 Jun 14. Allergy Asthma Proc. 2021. PMID: 34127176 Review.
-
The burden of hospitalizations and emergency department visits with hereditary angioedema and angioedema in the United States, 2007.Allergy Asthma Proc. 2010 Nov-Dec;31(6):511-9. doi: 10.2500/aap.2010.31.3403. Epub 2010 Oct 20. Allergy Asthma Proc. 2010. PMID: 20964950
-
Adherence and persistence among patients with hereditary angioedema receiving long-term prophylaxis in the United States.Allergy Asthma Proc. 2025 May 1;46(3):209-217. doi: 10.2500/aap.2025.46.250029. Epub 2025 Apr 29. Allergy Asthma Proc. 2025. PMID: 40300843
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

