Screening Anti-Parkinson's Disease Drugs in Living Mouse Brains via a Peroxynitrite-Activated Fluorescent Probe
- PMID: 40443559
- PMCID: PMC12117393
- DOI: 10.1021/cbmi.4c00076
Screening Anti-Parkinson's Disease Drugs in Living Mouse Brains via a Peroxynitrite-Activated Fluorescent Probe
Abstract
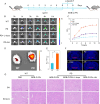
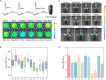
Screening anti-Parkinson's disease (PD) drugs at in vivo brain level is imperative for managing PD yet currently remains unaccomplished. Peroxynitrite (ONOO-) has been implicated in PD progression. Thus, developing in vivo ONOO--based imaging tools for anti-PD drug screening holds promise for early prognosis and treatment of PD. Consequently, a near-infrared (NIR) fluorescence probe, BOB-Cl-PN, with high specificity, good sensitivity (LOD = 24 nM), and rapid response (<60 s), was devised to investigate ONOO- and PD relationships. Utilizing NIR fluorescence imaging, BOB-Cl-PN effectively monitored ONOO- fluctuations in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced PD cell models, establishing a cellular high throughput screening (cHTS) system for anti-PD drugs. In live animal imaging, BOB-Cl-PN's ability to penetrate the blood-brain barrier enabled ONOO- flux imaging of PD mouse brains. Moreover, BOB-Cl-PN served as an imaging contrast for in vivo screening of potential traditional Chinese medicines for PD therapy, identifying fisetin as having the best therapeutic index among 10 Chinese medicines. This study constructs a sensitive, efficient imaging contrast for monitoring ONOO- dynamics in PD brains and provides a valuable platform for cellular and in vivo screening of anti-PD drugs.
Keywords: Chinese medicine; Parkinson’s disease; brain disease; fluorescent probe; peroxynitrite.
© 2024 The Authors. Co-published by Nanjing University and American Chemical Society.
Figures




References
LinkOut - more resources
Full Text Sources
Miscellaneous
