The translational power of Alzheimer's-based organoid models in personalized medicine: an integrated biological and digital approach embodying patient clinical history
- PMID: 40443709
- PMCID: PMC12119642
- DOI: 10.3389/fncel.2025.1553642
The translational power of Alzheimer's-based organoid models in personalized medicine: an integrated biological and digital approach embodying patient clinical history
Abstract
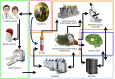
Alzheimer's disease (AD) is a complex neurodegenerative condition characterized by a multifaceted interplay of genetic, environmental, and pathological factors. Traditional diagnostic and research methods, including neuropsychological assessments, imaging, and cerebrospinal fluid (CSF) biomarkers, have advanced our understanding but remain limited by late-stage detection and challenges in modeling disease progression. The emergence of three-dimensional (3D) brain organoids (BOs) offers a transformative platform for bridging these gaps. BOs derived from patient-specific induced pluripotent stem cells (iPSCs) mimic the structural and functional complexities of the human brain. This advancement offers an alternative or complementary approach for studying AD pathology, including β-amyloid and tau protein aggregation, neuroinflammation, and aging processes. By integrating biological complexity with cutting-edge technological tools such as organ-on-a-chip systems, microelectrode arrays, and artificial intelligence-driven digital twins (DTs), it is hoped that BOs will facilitate real-time modeling of AD progression and response to interventions. These models capture central nervous system biomarkers and establish correlations with peripheral markers, fostering a holistic understanding of disease mechanisms. Furthermore, BOs provide a scalable and ethically sound alternative to animal models, advancing drug discovery and personalized therapeutic strategies. The convergence of BOs and DTs potentially represents a significant shift in AD research, enhancing predictive and preventive capacities through precise in vitro simulations of individual disease trajectories. This approach underscores the potential for personalized medicine, reducing the reliance on invasive diagnostics while promoting early intervention. As research progresses, integrating sporadic and familial AD models within this framework promises to refine our understanding of disease heterogeneity and drive innovations in treatment and care.
Keywords: Alzheimer’s disease; brain organoids; digital twins; early diagnosis biomarker; neurodegeneration; neuroinflammation; personalized medicine.
Copyright © 2025 Dolciotti, Righi, Grecu, Trucas, Maxia, Murtas and Diana.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Advanced biomarkers: Beyond amyloid and tau: Emerging non-traditional biomarkers for alzheimer`s diagnosis and progression.Ageing Res Rev. 2025 Jun;108:102736. doi: 10.1016/j.arr.2025.102736. Epub 2025 Mar 22. Ageing Res Rev. 2025. PMID: 40122399 Review.
-
Deciphering the role of lipid metabolism-related genes in Alzheimer's disease: a machine learning approach integrating Traditional Chinese Medicine.Front Endocrinol (Lausanne). 2024 Oct 23;15:1448119. doi: 10.3389/fendo.2024.1448119. eCollection 2024. Front Endocrinol (Lausanne). 2024. PMID: 39507054 Free PMC article.
-
Spheroid-Hydrogel-Integrated Biomimetic System: A New Frontier in Advanced Three-Dimensional Cell Culture Technology.Cells Tissues Organs. 2025;214(2):128-147. doi: 10.1159/000541416. Epub 2024 Sep 12. Cells Tissues Organs. 2025. PMID: 39265553 Free PMC article. Review.
-
iPSC-derived and Patient-Derived Organoids: Applications and challenges in scalability and reproducibility as pre-clinical models.Curr Res Toxicol. 2024 Oct 2;7:100197. doi: 10.1016/j.crtox.2024.100197. eCollection 2024. Curr Res Toxicol. 2024. PMID: 40276485 Free PMC article.
-
AI-driven innovations in Alzheimer's disease: Integrating early diagnosis, personalized treatment, and prognostic modelling.Ageing Res Rev. 2024 Nov;101:102497. doi: 10.1016/j.arr.2024.102497. Epub 2024 Sep 16. Ageing Res Rev. 2024. PMID: 39293530 Review.
References
-
- Aghdam M. A., Bozdag S., Saeed F. (2025). Alzheimer’s disease neuroimaging initiative. Machine-learning models for Alzheimer's disease diagnosis using neuroimaging data: survey, reproducibility, and generalizability evaluation. Brain Inform. 12:8. doi: 10.1186/s40708-025-00252-3, PMID: - DOI - PMC - PubMed
-
- Alić I., Goh P. A., Murray A., Portelius E., Gkanatsiou E., Gough G., et al. . (2021). Patient-specific Alzheimer-like pathology in trisomy 21 cerebral organoids reveals BACE2 as a gene dose-sensitive AD suppressor in human brain. Mol. Psychiatry 26, 5766–5788. doi: 10.1038/s41380-020-0806-5, PMID: - DOI - PMC - PubMed
-
- Allen A., Siefkas A., Pellegrini E., Burdick H., Barnes G., Calvert J., et al. . (2021). A digital twins machine learning model for forecasting disease progression in stroke patients. Appl. Sci. 11:5576. doi: 10.3390/app11125576 - DOI
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

