Lactylation: From Homeostasis to Pathological Implications and Therapeutic Strategies
- PMID: 40443721
- PMCID: PMC12122191
- DOI: 10.1002/mco2.70226
Lactylation: From Homeostasis to Pathological Implications and Therapeutic Strategies
Abstract
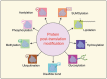
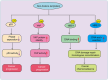
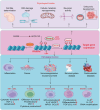
Lactylation, a recently identified post-translational modification, represents a groundbreaking addition to the epigenetic landscape, revealing its pivotal role in gene regulation and metabolic adaptation. Unlike traditional modifications, lactylation directly links metabolic intermediates, such as lactate, to protein function and cellular behavior. Emerging evidence highlights the critical involvement of lactylation in diverse biological processes, including immune response modulation, cellular differentiation, and tumor progression. However, its regulatory mechanisms, biological implications, and disease associations remain poorly understood. This review systematically explores the enzymatic and nonenzymatic mechanisms underlying protein lactylation, shedding light on the interplay between cellular metabolism and epigenetic control. We comprehensively analyze its biological functions in normal physiology, such as immune homeostasis and tissue repair, and its dysregulation in pathological contexts, including cancer, inflammation, and metabolic disorders. Moreover, we discuss advanced detection technologies and potential therapeutic interventions targeting lactylation pathways. By integrating these insights, this review aims to bridge critical knowledge gaps and propose future directions for research. Highlighting lactylation's multifaceted roles in health and disease, this review provides a timely resource for understanding its clinical implications, particularly as a novel target for precision medicine in metabolic and oncological therapies.
Keywords: biological significance; cancer immunotherapy; pathology; physiology; protein lactylation.
© 2025 The Author(s). MedComm published by Sichuan International Medical Exchange & Promotion Association (SCIMEA) and John Wiley & Sons Australia, Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Lactylation: The metabolic accomplice shaping cancer's response to radiotherapy and immunotherapy.Ageing Res Rev. 2025 Feb;104:102670. doi: 10.1016/j.arr.2025.102670. Epub 2025 Jan 24. Ageing Res Rev. 2025. PMID: 39864560 Review.
-
Lactylation's role in bone health and disease: mechanistic insights and therapeutic potential.PeerJ. 2025 Jun 9;13:e19534. doi: 10.7717/peerj.19534. eCollection 2025. PeerJ. 2025. PMID: 40511385 Free PMC article. Review.
-
Epigenetic Modulation by Lactylation in Sepsis: Linking Metabolism to Immune Dysfunction.J Inflamm Res. 2025 Jun 6;18:7357-7367. doi: 10.2147/JIR.S522081. eCollection 2025. J Inflamm Res. 2025. PMID: 40497149 Free PMC article. Review.
-
Mechanisms for Regulatory Effects of Exercise on Metabolic Diseases from the Lactate-Lactylation Perspective.Int J Mol Sci. 2025 Apr 8;26(8):3469. doi: 10.3390/ijms26083469. Int J Mol Sci. 2025. PMID: 40331975 Free PMC article. Review.
-
Lactylation: The emerging frontier in post-translational modification.Front Genet. 2024 Jun 27;15:1423213. doi: 10.3389/fgene.2024.1423213. eCollection 2024. Front Genet. 2024. PMID: 38993478 Free PMC article. Review.
References
-
- Ye L., Jiang Y., and Zhang M., “Crosstalk Between Glucose Metabolism, Lactate Production and Immune Response Modulation,” Cytokine & Growth Factor Reviews 68 (2022): 81–92. - PubMed
-
- Li X., Yu T., Li X., He X., Zhang B., and Yang Y., “Role of Novel Protein Acylation Modifications in Immunity and Its Related Diseases,” Immunology 173, no. 1 (2024): 53–75. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
