3D Printed Microfluidic Devices for Integrated Immunoaffinity Extraction, Solid-Phase Extraction, and Fluorescent Labeling of Preterm Birth Biomarkers
- PMID: 40443764
- PMCID: PMC12117449
- DOI: 10.1021/prechem.4c00092
3D Printed Microfluidic Devices for Integrated Immunoaffinity Extraction, Solid-Phase Extraction, and Fluorescent Labeling of Preterm Birth Biomarkers
Abstract
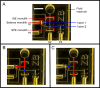
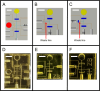
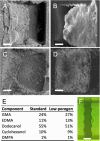
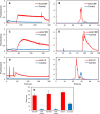
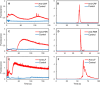
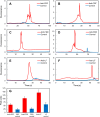
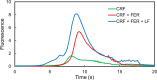
A miniaturized, biomarker-based diagnostic for preterm birth (PTB) risk will require multiple sample preparation steps to be integrated in a single platform. To this end, we created a 3D printed microfluidic device that combines immunoaffinity extraction (IAE), solid-phase extraction (SPE), and fluorescent labeling. This device uses an antibody-functionalized IAE monolith to selectively extract PTB biomarkers, a lauryl methacrylate reverse-phase SPE monolith to concentrate and facilitate fluorescent labeling of PTB biomarkers, and 3D printed valves to control flow through the monoliths. The advantageous iterative design process for arriving at a functional device is documented. The IAE/SPE device performed selective, reproducible extractions of three PTB biomarkers from buffer and depleted maternal blood serum, demonstrating its utility for single-biomarker and multiplexed extractions. After tandem extraction and fluorescent labeling, biomarkers eluted from the SPE monolith in a concentrated plug, facilitating future integration with downstream analysis techniques including microchip electrophoresis. This device effectively combines and automates orthogonal chromatographic extraction methods and constitutes a substantial step toward a complete microfluidic PTB prediction platform.
Keywords: 3D printing; lab-on-a-chip; microfluidics; molecular diagnostics; monoliths; point-of-care diagnostics.
© 2025 The Authors. Co-published by University of Science and Technology of China and American Chemical Society.
Figures
References
-
- Ohuma E. O., Moller A.-B., Bradley E., Chakwera S., Hussain-Alkhateeb L., Lewin A., Okwaraji Y. B., Mahanani W. R., Johansson E. W., Lavin T.. et al. National, regional, and global estimates of preterm birth in 2020, with trends from 2010: a systematic analysis. Lancet. 2023;402(10409):1261–1271. doi: 10.1016/S0140-6736(23)00878-4. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
