Decoupling Global and Local Structural Changes in Self-aminoacylating Ribozymes Reveals the Critical Role of Local Structural Dynamics in Ribozyme Activity
- PMID: 40443886
- PMCID: PMC12117395
- DOI: 10.1021/jacsau.5c00146
Decoupling Global and Local Structural Changes in Self-aminoacylating Ribozymes Reveals the Critical Role of Local Structural Dynamics in Ribozyme Activity
Abstract
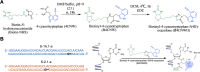
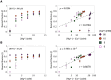
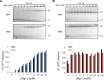
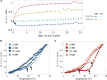
Self-aminoacylating ribozymes catalyze the attachment of amino acids to RNA, serving as pivotal models to investigate the catalytic roles of RNA in prebiotic evolution. In this study, we investigated how divalent metal ions (Mg2+ and Ca2+) modulate local and global structures in two such ribozymes, S-1A.1-a and S-2.1-a, using 4-cyanotryptophan (4CNW) fluorescence and native gel electrophoresis. By tracking 4CNW fluorescence changes at varying concentrations of Mg2+ and Ca2+ and temperatures, we determined how these ions influence the catalytic sites and overall conformations of the ribozymes. Our findings reveal that Mg2+ specifically binds to S-1A.1-a at low concentrations, stabilizing the local structure around the aminoacylation site and causing the site to become more buried, which is essential for catalytic activity. Although higher Mg2+ and Ca2+ concentrations induce global structural rearrangements, these shifts have minimal impact on the local environment of the aminoacylation site, underscoring the dominance of local structural stability in sustaining ribozyme function. In contrast, the activity of S-2.1-a effectively adapts to both Mg2+ and Ca2+, and its fluorescence results indicate a more solvent-exposed aminoacylation site. Overall, these data highlight that local structural changes in the ribozyme's catalytic core are more critical for its function than global conformational shifts. Our study highlights the importance of local environmental changes in ion-dependent ribozyme catalysis and provides insights into the molecular mechanisms of self-aminoacylating ribozymes.
Keywords: 4-cyanotryptophan (4CNW) fluorescence; divalent metal ions; local and global structural dynamics; ribozyme catalysis; self-aminoacylating ribozymes.
© 2025 The Authors. Published by American Chemical Society.
Figures


Similar articles
-
Mechanistic characterization of the HDV genomic ribozyme: assessing the catalytic and structural contributions of divalent metal ions within a multichannel reaction mechanism.Biochemistry. 2001 Oct 9;40(40):12022-38. doi: 10.1021/bi011253n. Biochemistry. 2001. PMID: 11580278
-
Catalytic cleavage of cis- and trans-acting antigenomic delta ribozymes in the presence of various divalent metal ions.Nucleic Acids Res. 2001 Nov 1;29(21):4482-92. doi: 10.1093/nar/29.21.4482. Nucleic Acids Res. 2001. PMID: 11691936 Free PMC article.
-
Emergent properties as by-products of prebiotic evolution of aminoacylation ribozymes.Nat Commun. 2022 Jun 25;13(1):3631. doi: 10.1038/s41467-022-31387-0. Nat Commun. 2022. PMID: 35752631 Free PMC article.
-
Two distinct catalytic strategies in the hepatitis δ virus ribozyme cleavage reaction.Biochemistry. 2011 Nov 8;50(44):9424-33. doi: 10.1021/bi201157t. Epub 2011 Oct 17. Biochemistry. 2011. PMID: 22003985 Free PMC article. Review.
-
The Varkud Satellite Ribozyme: A Thirty-Year Journey through Biochemistry, Crystallography, and Computation.Acc Chem Res. 2021 Jun 1;54(11):2591-2602. doi: 10.1021/acs.accounts.1c00052. Epub 2021 May 11. Acc Chem Res. 2021. PMID: 33974386 Review.
References
LinkOut - more resources
Full Text Sources
Miscellaneous
