The effects of a semen cuscutae flavonoids-based antidepressant treatment on microbiome and metabolome in mice
- PMID: 40444002
- PMCID: PMC12119544
- DOI: 10.3389/fmicb.2025.1558833
The effects of a semen cuscutae flavonoids-based antidepressant treatment on microbiome and metabolome in mice
Abstract
Background: Depression is a prevalent psychiatric disorder and one of the leading causes of disability worldwide. Previous studies have shown that Semen Cuscutae flavonoids (SCFs) exert antidepressant effects by modulating the microbiota-neuroinflammation axis and ameliorating hippocampal metabolic disturbances. However, the impact of SCFs on gut microbiota and related metabolomics remains largely undefined. Given that the gut microbiota has been proven to play a significant role in the etiology of depression and serves as a promising target for its treatment in humans, this study aims to elucidate the antidepressant effects of SCFs and to investigate how they modulate microbial and metabolic pathways to alleviate depressive symptoms.
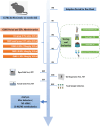
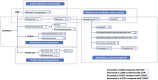
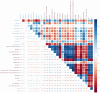
Materials and methods: Chronic unpredictable mild stress (CUMS)-induced mice were used as a depression model. The normal mice and CUMS-induced mice were treated with either vehicle or with SCFs. A range of standardized behavioral assays and physiological indicators were employed to evaluate the antidepressant effects of SCFs. Upon the confirmation of the effectiveness of the SCFs treatment, the composition, richness, and diversity of the fecal microbiota were assessed using 16S rRNA gene sequencing. Additionally, fecal metabolic profiling was analyzed using UHPLC-MS/MS-based metabolomics. Multivariate data analysis was subsequently performed to identify differential metabolites and characterize alterations in fecal metabolites. Furthermore, a correlation analysis between differential metabolites and key microbiota was conducted.
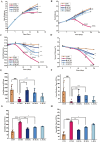
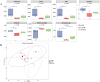
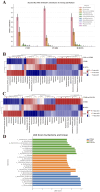
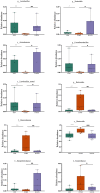
Results: SCFs significantly ameliorated depressive behaviors and the dysregulated diversity of fecal microbiota induced by CUMS. SCFs enhanced the gut microbiota structure in the CUMS group by increasing the Firmicutes/Bacteroidota ratio, significantly elevating the abundance of Firmicutes, Lactobacillus, Limosilactobacillus, and Actinobacteria while reducing the abundance of Bacteroidota and Bacteroides in CUMS-treated mice. Fecal metabolomics analyses revealed that SCFs could modulate metabolic pathways, including aldosterone synthesis and secretion, arachidonic acid metabolism, and primary bile acid biosynthesis.
Conclusions: Mice with depression induced by CUMS exhibited disturbances in both their gut microbiota and fecal metabolism. However, SCFs restored the balance of the microbial community and corrected metabolic disturbances in feces, exerting antidepressant effects through a multifaceted mechanism.
Keywords: CUMS; SCFs; depression; feces; metabolites; microbiota.
Copyright © 2025 Shao, Zhou, Li, Jin, Fu, Liu, Wang, Du and Chen.
Conflict of interest statement
SZ was employed by Qingdao Ruyi Software Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Semen Cuscutae flavonoids activated the cAMP-PKA-CREB-BDNF pathway and exerted an antidepressant effect in mice.Front Pharmacol. 2024 Nov 25;15:1491900. doi: 10.3389/fphar.2024.1491900. eCollection 2024. Front Pharmacol. 2024. PMID: 39654620 Free PMC article.
-
Integrated 16S rRNA sequencing and metabolomics analysis to investigate the antidepressant role of Yang-Xin-Jie-Yu decoction on microbe-gut-metabolite in chronic unpredictable mild stress-induced depression rat model.Front Pharmacol. 2022 Sep 30;13:972351. doi: 10.3389/fphar.2022.972351. eCollection 2022. Front Pharmacol. 2022. PMID: 36249818 Free PMC article.
-
Impact of traditional Chinese medicine treatment on chronic unpredictable mild stress-induced depression-like behaviors: intestinal microbiota and gut microbiome function.Food Funct. 2019 Sep 1;10(9):5886-5897. doi: 10.1039/c9fo00399a. Epub 2019 Aug 29. Food Funct. 2019. PMID: 31464319
-
A combination of cecum microbiome and metabolome in CUMS depressed rats reveals the antidepressant mechanism of traditional Chinese medicines: A case study of Xiaoyaosan.J Ethnopharmacol. 2021 Aug 10;276:114167. doi: 10.1016/j.jep.2021.114167. Epub 2021 May 10. J Ethnopharmacol. 2021. PMID: 33984458
-
Deciphering the therapeutic potential of SheXiangXinTongNing: Interplay between gut microbiota and brain metabolomics in a CUMS mice model, with a focus on tryptophan metabolism.Phytomedicine. 2024 Jul;129:155584. doi: 10.1016/j.phymed.2024.155584. Epub 2024 Apr 20. Phytomedicine. 2024. PMID: 38704913
References
-
- Bao Y., Shen Y., Wu Z., Tao S., Yang B., Zhu T., et al. . (2023). High dietary arachidonic acid produces excess eicosanoids, and induces hepatic inflammatory responses, oxidative stress and apoptosis in juvenile Acanthopagrus schlegelii. Aquacult. Rep. 29:101506. 10.1016/j.aqrep.2023.101506 - DOI
LinkOut - more resources
Full Text Sources

