Proteomic Characterization of Extracellular Vesicles from Human Neural Precursor Cells: A Promising Advanced Therapy for Neurodegenerative Diseases
- PMID: 40444011
- PMCID: PMC12121667
- DOI: 10.2147/IJN.S502031
Proteomic Characterization of Extracellular Vesicles from Human Neural Precursor Cells: A Promising Advanced Therapy for Neurodegenerative Diseases
Abstract
Background: The therapeutic effect of stem cells is attributed to their direct maturation into somatic cells and their paracrine effects, which influence the extracellular environment. One such component released is extracellular vesicles containing proteins and genetic materials with immunomodulatory functions and facilitating cell-to-cell communication.
Purpose: The study's main objective was to characterize extracellular vesicles (EVs) from Human Neural Precursor Cells (hNPCs).
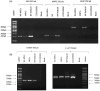
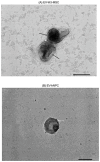
Methods: Wharton's Jelly mesenchymal stem cells (WJ-MSCs) were isolated by explant technique and characterized by flow cytometry and trilineage differentiation. The hNPCs obtained from neurospheres were produced by seeding WJ-MSCs on a natural functional biopolymer matrix. EVs derived from WJ-MSCs and hNPCs were isolated by precipitation methodology and characterized by flow cytometry, nanoparticle tracking analysis (NTA), scanning electron microscopy (TEM), and proteomic.
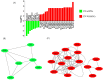
Results: hNPCs expressed proteins and genes characteristic of neural precursor cells. The EVs were characterized by flow cytometry and showed varied expression for the markers CD63, CD9, and CD81, indicating different subpopulations based on their origin of formation. NTA and TEM of the EVs exhibited characteristic size, shape, and structural integrity consistent with the criteria established by the International Society for Extracellular Vesicles (ISEV). EV-hNPCs function enrichment analysis of the proteomic results showed that these vesicles presented abundant proteins directly involved in neuronal biological processes such as plasticity, transduction, postsynaptic density, and overall brain development.
Discussion: The results indicate that EVs derived from hNPCs maintain key neural precursor characteristics and exhibit marker variability, suggesting distinct subpopulations. Their structural integrity aligns with ISEV standards, supporting their potential as reliable biological entities. The proteomic analysis highlights their role in neuronal functions, reinforcing their applicability in neurodegenerative research and therapeutic strategies.
Conclusion: The EVs were successfully isolated from hNPCs with abundant proteins involved in neuronal processes, making them attractive for acellular therapies to treat neurodegenerative diseases.
Keywords: Wharton’s Jelly; extracellular vesicles; mesenchymal stem cell; neural precursor cells; neurospheres.
© 2025 Stricker et al.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures








References
-
- Majors S. Regenerative medicine: the pipeline momentum builds H1. Alliance Regener Med. 2022;2022:1–30.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

