The Impact of Ligand Oxidation State and Fold Angle on the Charge Transfer Processes of MoIVO-Dithione Complexes
- PMID: 40444014
- PMCID: PMC12121939
- DOI: 10.1002/ejic.202001155
The Impact of Ligand Oxidation State and Fold Angle on the Charge Transfer Processes of MoIVO-Dithione Complexes
Abstract
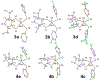
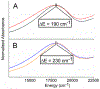
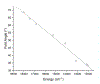
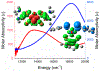
We report a series of mononuclear monooxo Mo(IV) complexes possessing either one or two fully oxidized dithiolene ligands; [MoOCl(R2Dt0)2][X], (1 and 2), and MoO(p-SC6H4Y)2(R2Dt0), (3 and 4), (R=Me, i Pr; X= PF6, SbF6, BF4; Y= H, Cl, F, CF3, Me, t Bu, OMe). Either four or two quasi-reversible ligand-based redox couples are detected depending upon the number of fully oxidized dithiolene ligands present. The molecular structure of 3 and 4 exhibit a large (47° to 70°) fold angle along the S•••S vector of the dithione ligand. The UV-Vis spectra of 3 and 4 exhibit a low energy charge transfer band at ~540 nm that are red-shifted ~200 nm compared to the spectra of 1 and 2. Density Functional Theory (DFT) calculations show that the low energy charge transfer band of 3 and 4 is heavily influenced by ligand fold angle. Reducing the fold angle decreases the charge transfer energy, and the transition becomes more ligand-based.
Keywords: Charge Transfer; Dithiolene; Donor-Acceptor; Fold Angle; Molybdenum.
Figures
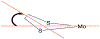
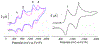
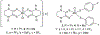
References
-
- Schrauzer GN, Mayweg V, J. Am. Chem. Soc 1962, 3221;
- Davison A, Edelstein N, Holm RH, Maki AH, J. Am. Chem. Soc 1963, 85, 3049–3050;
- Davison A, Edelstein N, Holm RH, Maki AH, Inorg. Chem 1963, 2, 1227–1232;
- Davison A, Edelstein N, Holm RH, Maki AH, J. Am. Chem. Soc 1963, 85, 2029–2030;
- Maki AH, Edelstein N, Davison A, Holm RH, J. Am. Chem. Soc 1964, 86, 4580–4587;
- Balch AL, Röhrscheid F, Holm RH, J. Am. Chem. Soc 1965, 87, 2301–2302;
- Stiefel EI, Waters JH, Billig E, Gray HB, J. Am. Chem. Soc 1965, 87, 3016–3017;
- Sproules S, Benedito F. v. L., Bill E, Weyhermüller T, DeBeer George S, Wieghardt K, Inorg. Chem 2009, 48, 10926–10941; - PubMed
- Ghosh M, Weyhermueller T, Wieghardt K, Dalton Trans. 2010, 39, 1996–2007. - PubMed
-
- Mogesa B, Perera E, Rhoda HM, Gibson JK, Oomens J, Berden G, van Stipdonk MJ, Nemykin VN, Basu P, Inorg. Chem 2015, 54, 7703–7716; - PMC - PubMed
- Yang J, Mogesa B, Basu P, Kirk ML, Inorg. Chem 2016, 55, 785–793; - PMC - PubMed
- Mtei RP, Perera E, Mogesa B, Stein B, Basu P, Kirk ML, Eur. J. Inorg. Chem 2011, 2011, 5467–5470; - PMC - PubMed
- Perera E, Basu P, Dalton Trans. 2009, 5023–5028; - PMC - PubMed
- Deplano P, Pilia L, Espa D, Mercuri ML, Serpe A, Coord. Chem. Rev 2010, 254, 1434–1447;
- Espa D, Pilia L, Makedonas C, Marchio L, Mercuri ML, Serpe A, Barsella A, Fort A, Mitsopoulou CA, Deplano P, Inorg. Chem 2014, 53, 1170–1183; - PubMed
- Espa D, Pilia L, Marchio L, Mercuri ML, Serpe A, Barsella A, Fort A, Dalgleish SJ, Robertson N, Deplano P, Inorg. Chem 2011, 50, 2058–2060; - PubMed
- Pilia L, Espa D, Barsella A, Fort A, Makedonas C, Marchio L, Mercuri ML, Serpe A, Mitsopoulou CA, Deplano P, Inorg. Chem 2011, 50, 10015–10027; - PubMed
- Basu P, Nigam A, Mogesa B, Denti S, Nemykin VN, Inorg. Chim. Acta 2010, 363, 2857–2864. - PMC - PubMed
-
- Ratvasky SC, Mogesa B, van Stipdonk MJ, Basu P, Polyhedron 2016, 114, 370–377; - PMC - PubMed
- Colston KJ, Dille SA, Mogesa B, Astashkin AV, Brant JA, Zeller M, Basu P, Eur. J. Inorg. Chem 2019, 2019, 4939–4948;
- Nemykin VN, Olsen JG, Perera E, Basu P, Inorg. Chem 2006, 45, 3557–3568; - PubMed
- Colston KJ, Dille SA, Mogesa B, Brant J, Nemykin VN, Zeller M, Basu P, RCS Advances 2020, 10, 38294–38303. - PMC - PubMed
-
- Attar SS, Marchio L, Pilia L, Casula MF, Espa D, Serpe A, Pizzotti M, Marinotto D, Deplano P, New J. Chem 2019, 43, 12570–12579;
- Attar S, Espa D, Artizzu F, Pilia L, Serpe A, Pizzotti M, Di Carlo G, Marchio L, Deplano P, Inorg. Chem 2017, 56, 6763–6767; - PubMed
- Pilia L, Espa D, Concas G, Congiu F, Marchio L, Laura Mercuri M, Serpe A, Deplano P, New J. Chem 2015, 39, 4716–4725;
- Espa D, Pilia L, Marchio L, Artizzu F, Serpe A, Mercuri ML, Simao D, Almeida M, Pizzotti M, Tessore F, Deplano P, Dalton Trans. 2012, 41, 3485–3493. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
