Comparative safety analysis of bevacizumab and alkylating agent in glioblastoma management - What have we learned recently?
- PMID: 40444037
- PMCID: PMC12120556
- DOI: 10.3389/fphar.2025.1595642
Comparative safety analysis of bevacizumab and alkylating agent in glioblastoma management - What have we learned recently?
Abstract
Objective: Alkylating agents and bevacizumab are both first-line chemotherapeutic options for the treatment of glioblastoma; however, their mechanisms of action differ substantially. This study aimed to compare the safety profiles of these two drug classes in the treatment of glioblastoma to inform clinical decision-making.
Methods: Adverse events reported between the first quarter of 2004 and the fourth quarter of 2023 were analyzed using data from the FDA Adverse Event Reporting System (FAERS) database. Disproportionality analysis was employed to assess and compare the AE signals associated with bevacizumab and alkylating agents.
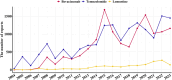
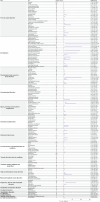
Results: In the context of glioblastoma treatment, 3,323 adverse reports were associated with bevacizumab, 5,283 with temozolomide, and 427 with lomustine. The most frequently reported AEs for bevacizumab were fatigue (n = 276), hypertension (n = 220), and headache (n = 199). Compared to temozolomide, bevacizumab was more strongly associated with "vascular disorders," "renal and urinary disorders," and "hypertension." Notably, bevacizumab appeared to offer a potential safety advantage with respect to hematological adverse events.
Conclusion: Our analysis indicates that bevacizumab exhibits a distinct safety profile compared to alkylating agents, particularly demonstrating a lower incidence of hematological adverse events. Further prospective studies are warranted to validate these findings and to elucidate the underlying mechanisms responsible for the observed adverse events.
Keywords: FAERS; alkylating agent; bevacizumab; glioblastoma; safety profile; temozolomide.
Copyright © 2025 Qu, Zhao, Yang, Fu, Bai, Li and Wang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Australian Government Department of Health and Aged Care (2019). Public summary document – may 2019 PBAC meeting. Available online at: https://www.pbs.gov.au/pbs/industry/listing/elements/pbac-meetings/psd/p... (Accessed June 21, 2023).
LinkOut - more resources
Full Text Sources

