Involvement of EGFR-AKT signaling in hemin-induced neurotoxicity
- PMID: 40444141
- PMCID: PMC12121490
- DOI: 10.3389/ebm.2025.10554
Involvement of EGFR-AKT signaling in hemin-induced neurotoxicity
Abstract
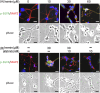
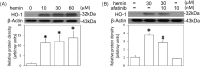
Intracerebral hemorrhage (ICH), as bleeding from ruptured vessels within the brain, is the second leading neuropathological problem following ischemic stroke. In the present study, the involvement of epithelial growth factor receptor (EGFR)-tyrosine kinase (TK) signaling underlying ICH-related neurodegeneration was investigated using afatinib, a clinically available EGFR-tyrosine kinase inhibitor (EGFR-TKI). We employed hemin (a breakdown product of hemoglobin) to mimic the pathophysiology of ICH in primary cultured cortical neurons. Using a lactate dehydrogenase (LDH) assay, incubation of hemin concentration- and time-dependently induced neuronal death. Simultaneous incubation of afatinib (10 nM) significantly inhibited hemin (30 μM)-induced neuronal death. Immunofluorescent data demonstrated that co-treatment of afatinib for 1 h attenuated hemin (30 μM)-induced elevation in phosphorylated-EGFR (p-EGFR) immunoreactivity and neurite impairment. Western blot assay demonstrated that co-incubation of afatinib for 16 h diminished hemin-induced elevation in p-EGFR and p-AKT, tumor necrosis factor-α and cyclooxygenase 2 (two proinflammatory biomarkers) as well as heme oxygenase-1 (HO-1, an enzyme catalyzing heme/hemin), glutathione hydroperoxidase 4 and receptor-interacting protein 3 (two biomarkers of ferroptosis and necroptosis). In addition, co-treatment of afatinib for 24 h inhibited hemin-induced NO production in the culture medium. In conclusion, our study shows that afatinib via blocking EGFR-AKT signaling inhibits hemin-induced EGFR-AKT activation, neuroinflammation, HO-1 expression and programed cell death, suggesting that EGFR-AKT signaling is involved in hemin-induced neurotoxicity and may be a druggable target for ICH.
Keywords: EGFR-AKT signaling; afatinib; ferroptosis; hemin; primary cultured cortical neurons.
Copyright © 2025 Huang, Tseng, Lee, Lo and Lin.
Conflict of interest statement
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures
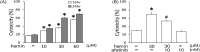



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

