Methamphetamine exposure during gestation and lactation periods impairs the learning and memory of offspring mice, which is reversed by melatonin: the role of oxidative stress and acetylcholinesterase
- PMID: 40444166
- PMCID: PMC12118776
- DOI: 10.4103/RPS.RPS_187_23
Methamphetamine exposure during gestation and lactation periods impairs the learning and memory of offspring mice, which is reversed by melatonin: the role of oxidative stress and acetylcholinesterase
Abstract
Background and purpose: Melatonin is a product of the pineal gland, which regulates the circadian cycle. Neurotoxicity is the most important side effect of methamphetamine (Met) abuse during pregnancy. This study aimed to explore the effect of Met exposure during gestation and lactation periods on the learning and memory of offspring mice. The protective effect of melatonin and the role of oxidative stress and acetylcholinesterase were also investigated.
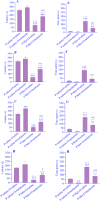
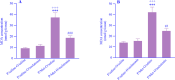
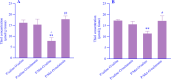
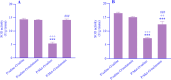
Experimental approach: The pregnant mice were randomly divided into 2 groups. Saline or Met (5 mg/kg) was injected daily during pregnancy and lactation. After the lactation period, the offspring mice of each group were divided into 2 subgroups, and saline or melatonin (10 mg/kg) was orally (gavage) administered to the offspring mice from the post-delivery (PD) day 21 up to PD Day 60. The offspring mice were examined in the passive avoidance (PA) test. Finally, oxidative stress markers and acetylcholinesterase (AchE) activity were measured in the brains.
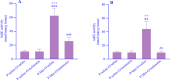
Findings/results: As a result, Met decreased delay and light time while increasing the frequency of entry and time in the dark region of PA. However, melatonin alleviated the impairing effect of Met on PA performance. Meanwhile, the administration of Met increased malondialdehyde while decreasing superoxide dismutase and thiol content. Furthermore, AchE activity was significantly increased in Met-treated mice. Melatonin reversed the levels of antioxidants, lipid peroxidation, and AchE activity in the brain.
Conclusion and implications: Together, these results suggested that melatonin may be a potential therapeutic agent for alleviating Met-induced memory impairment by restoring redox hemostasis and AchE.
Keywords: Acetylcholinesterase; Melatonin; Methamphetamine; Oxidative stress.
Copyright: © 2025 Research in Pharmaceutical Sciences.
Conflict of interest statement
All authors declared no conflict of interest in this study.
Figures

Similar articles
-
The therapeutic effect of melatonin on female offspring ovarian reserve and quality in BALB/c mice after exposing their mother to methamphetamine during pregnancy and lactation.Iran J Basic Med Sci. 2023 Feb;26(2):208-215. doi: 10.22038/IJBMS.2022.66660.14636. Iran J Basic Med Sci. 2023. PMID: 36742138 Free PMC article.
-
Effect of melatonin on male offspring testis and sperm parameters in BALB/c mice after exposing their mother to METHamphetamine during pregnancy and lactation.Iran J Basic Med Sci. 2023;26(7):777-784. doi: 10.22038/IJBMS.2023.69608.15158. Iran J Basic Med Sci. 2023. PMID: 37396947 Free PMC article.
-
The absence of maternal pineal melatonin rhythm during pregnancy and lactation impairs offspring physical growth, neurodevelopment, and behavior.Horm Behav. 2018 Sep;105:146-156. doi: 10.1016/j.yhbeh.2018.08.006. Epub 2018 Sep 1. Horm Behav. 2018. PMID: 30114430
-
Melatonin reverses cognitive deficits in streptozotocin-induced type 1 diabetes in the rat through attenuation of oxidative stress and inflammation.J Chem Neuroanat. 2021 Mar;112:101902. doi: 10.1016/j.jchemneu.2020.101902. Epub 2020 Dec 1. J Chem Neuroanat. 2021. PMID: 33276072
-
Effect of insulin and melatonin on acetylcholinesterase activity in the brain of amnesic mice.Behav Brain Res. 2008 Jun 3;189(2):381-6. doi: 10.1016/j.bbr.2008.01.015. Epub 2008 Feb 5. Behav Brain Res. 2008. PMID: 18336929
References
-
- Prakash MD, Tangalakis K, Antonipillai J, Stojanovska L, Nurgali K, Apostolopoulos V. Methamphetamine: effects on the brain, gut and immune system. Pharmacol Res. 2017;120:60–67. DOI: 10.1016/j.phrs.2017.03.009. - PubMed
-
- Baei F, Rajabzadeh A, Bagheri J, Jalayeri Z, Ebrahimzadeh-Bideskan A. Effect of methamphetamine exposure during pregnancy and lactation on polysialic acid-neural cell adhesion molecule expression in rat’s offspring hippocampus. Metab Brain Dis. 2017;32(4):991–1002. DOI: 10.1007/s11011-017-9973-8. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
