Cherenkov light emission in external beam radiation therapy of the larynx
- PMID: 40444263
- PMCID: PMC12120355
- DOI: 10.1117/1.JBO.30.5.055002
Cherenkov light emission in external beam radiation therapy of the larynx
Abstract
Significance: Cherenkov light emitted in the tissue during radiation therapy enables unprecedented approaches to tumor functional imaging for early treatment assessment. Cherenkov light-based tomographic imaging requires image reconstruction algorithms based on internal light sources that, in turn, require knowledge about the characteristics of the Cherenkov light within the patient.
Aim: We aim to investigate the spatial and spectral characteristics of Cherenkov light within the patient and at the patient's surface, and the origin within the tissue of light reaching the surface, to provide insight for the development of image reconstruction algorithms for Cherenkov light-based tomographic imaging.
Approach: Numerical experiments using clinical patient data and Monte Carlo simulations are performed for the radiation therapy of laryngeal cancer for intensity-modulated radiation therapy and volumetric-modulated arc radiation therapy.
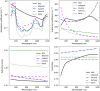
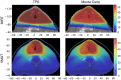
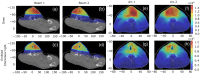
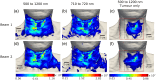
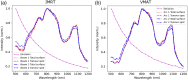
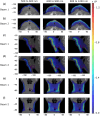
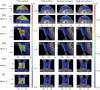
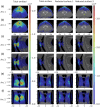
Results: The emitted Cherenkov light is concentrated in regions of high delivered dose, with the spatial distribution within the patient and at the patient's surface depending on the treatment type and patient anatomy. The Cherenkov light at the patient's surface is dominant in the near-infrared spectral region. Light emitted within the tumor emerges at the patient's surface on a well-defined radiation beam-independent region. The distribution within the patient of the emitted light that emerges on reduced areas on the patient's surface containing this region is similar to that of the light that emerges across the entire patient's surface.
Conclusions: Detailed information about the spectral and spatial characteristics of Cherenkov light is provided. In addition, these results suggest that surface light measurements restricted to smaller areas containing the region where the light emitted in the tumor emerges (that can be determined through simulations prior to the treatment) could enable probing the tumor while being easier to integrate with the radiotherapy system and while the effect of measurement data incompleteness on image reconstruction may not be too strong.
Keywords: Cherenkov light; radiation therapy; spatial and spectral characteristics.
© 2025 The Authors.
Figures



References
-
- Frank I., Tamm I., “Coherent visible radiation of fast electrons passing through matter,” in Selected Papers, Bolotovskii B. M., et al., Eds., pp. 29–35, Springer; (1991).
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources

