Monthly pulse methylprednisolone infusions in patients with non-idiopathic pulmonary fibrosis interstitial lung diseases: a single-center retrospective analyses
- PMID: 40444328
- PMCID: PMC12126682
- DOI: 10.1177/17534666251342661
Monthly pulse methylprednisolone infusions in patients with non-idiopathic pulmonary fibrosis interstitial lung diseases: a single-center retrospective analyses
Abstract
Background: Non-idiopathic pulmonary fibrosis interstitial lung diseases (non-IPF ILDs) comprise a broad spectrum of pathologies with varying degrees of inflammation and fibrosis. Progressive fibrosing ILD is associated with significant mortality and limited treatment options. Standard regimens employ multimodal immunosuppression, most commonly prolonged courses of oral corticosteroids (OCS), that are associated with a high risk of adverse effects and limited proven efficacy.
Objectives: This study investigates the safety, tolerability, and effectiveness of monthly intravenous pulse methylprednisolone (PMP) for the treatment of patients with progressive non-IPF ILD.
Design: Retrospective single-center cohort study of patients at an academic tertiary referral center for ILD between October 2019 and September 2022.
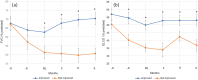
Methods: All non-IPF ILD patients who received intravenous PMP (1000 mg daily for three consecutive days/month) between October 2019 and September 2022 were included. The decision to treat was based on a multidisciplinary consensus diagnosis following ATS/ERS/JRS/ALAT guidelines and confirmed or at high risk for ILD progression. Treatment continuation was contingent upon pulmonary function test (PFT) improvement (assessed approximately every 3 months), tolerable adverse events, and shared decision making with patients. Effectiveness was measured by a change in forced vital capacity (FVC) and diffusion limit of carbon monoxide (DLCO), with improvement being defined as an absolute increase in either FVC >5% or DLCO >10% from baseline.
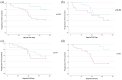
Results: Thirty-three patients received PMP at our center. One patient died of an acute exacerbation of ILD. Of the 32 patients included for analysis, 17 (53%) exhibited improved lung function with PMP between PFTs, which was maintained for a median follow-up of 209 days. The regimen was generally well-tolerated, with the most common adverse effects being insomnia and restlessness on infusion days. Advanced disease, indicated by lower FVC, traction bronchiectasis, and oxygen dependence, predicted poor response.
Conclusions: PMP may offer a safer, better-tolerated, and more effective treatment for progressive non-IPF ILD than prolonged OCS. Notably, a third of fibrotic hypersensitivity pneumonitis patients showed improved FVC after 3 months of PMP, defying expectations of steroid non-responsiveness. However, further well-designed controlled prospective clinical trials are needed to confirm our findings and establish long-term safety.
Keywords: corticosteroids; interstitial lung disease; non-IPF; progressive pulmonary fibrosis; pulse corticosteroids.
Plain language summary
Pulse Methylprednisolone for Progressive Non-IPF ILD - a potential alternative to oral steroidsNon-Idiopathic Pulmonary Fibrosis interstitial lung diseases (non-IPF ILDs) are a broad group of diseases with inflammation and scarring of the lungs. Current treatment centers on oral corticosteroids (primarily prolonged prednisone) despite limited evidence and significant adverse events. This retrospective study reports our experience with using monthly pulse methylprednisolone (PMP) and contributes to the search for identifying treatable traits in non-IPF ILDs. 53% of the patients who received PMP had significant improvement in ILD, and there were well tolerate side- effects during treatments. We also identify traction bronchiectasis and home oxygen use as negative prognostic factor for response to steroids. This pilot study lays the foundation for future trials of pulse steroids in non-IPF ILDs as a potential alternative to prolonged oral corticosteroids.
Figures
Similar articles
-
Pirfenidone in patients with progressive fibrotic interstitial lung diseases other than idiopathic pulmonary fibrosis (RELIEF): a double-blind, randomised, placebo-controlled, phase 2b trial.Lancet Respir Med. 2021 May;9(5):476-486. doi: 10.1016/S2213-2600(20)30554-3. Epub 2021 Mar 30. Lancet Respir Med. 2021. PMID: 33798455 Clinical Trial.
-
Possible value of antifibrotic drugs in patients with progressive fibrosing non-IPF interstitial lung diseases.BMC Pulm Med. 2019 Nov 12;19(1):213. doi: 10.1186/s12890-019-0937-0. BMC Pulm Med. 2019. PMID: 31718637 Free PMC article.
-
Real-life experiences in a single center: efficacy of pirfenidone in idiopathic pulmonary fibrosis and fibrotic idiopathic non-specific interstitial pneumonia patients.Ther Adv Respir Dis. 2020 Jan-Dec;14:1753466620963015. doi: 10.1177/1753466620963015. Ther Adv Respir Dis. 2020. PMID: 33070705 Free PMC article.
-
Nintedanib: A Review in Fibrotic Interstitial Lung Diseases.Drugs. 2021 Apr;81(5):575-586. doi: 10.1007/s40265-021-01487-0. Epub 2021 Mar 25. Drugs. 2021. PMID: 33765296 Free PMC article. Review.
-
A cohort study of Danish patients with interstitial lung diseases: burden, severity, treatment and survival.Dan Med J. 2015 Apr;62(4):B5069. Dan Med J. 2015. PMID: 25872544 Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

