ESPGHAN/NASPGHAN guidelines for treatment of irritable bowel syndrome and functional abdominal pain-not otherwise specified in children aged 4-18 years
- PMID: 40444524
- PMCID: PMC12314588
- DOI: 10.1002/jpn3.70070
ESPGHAN/NASPGHAN guidelines for treatment of irritable bowel syndrome and functional abdominal pain-not otherwise specified in children aged 4-18 years
Abstract
Objectives: Abdominal pain related disorders of gut-brain interaction (AP-DGBIs) such as irritable bowel syndrome (IBS) and functional abdominal pain-not otherwise specified (FAP) are common conditions in children, significantly impacting quality of life. This treatment guideline for IBS and FAP in children of 4-18 years is a collaborative effort of the European and North American Societies for Pediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN and NASPGHAN). We aim to comprehensively review the current evidence on treatment options and offer evidence-based recommendations with utility across all treatment settings worldwide, as well as to provide methodological directions for future research.
Methods: The guideline development followed the "Grading of Recommendations Assessment, Development and Evaluation" (GRADE) approach, which is in accordance with the GRADE handbook and supported by the World Health Organization. The Guideline Development Group (GDG) comprised clinical experts, representing ESPGHAN, NASPGHAN, and Cochrane. Individual members have put forward a final consensus list of treatment options, which were then translated into "patient, intervention, comparison, outcome" (PICO) format options. Prospective agreement on decision thresholds for efficacy and safety outcomes was reached through a Delphi process among the GDG to support GRADEing of the literature. Consensus voting was used to finalize recommendations, and a treatment algorithm was developed.
Results: Systematic literature searches for this output identified 86 original randomized controlled trials assessing treatment of IBS and FAP. Consensus was reached for 25 GRADEd recommendations. Ten best practice statements were formulated, and guidance for future research methodology was proposed.
Conclusion: This guideline represents the first collaborative output of ESPGHAN and NASPGHAN on treatment options for AP-DGBIs. Systematic review of the evidence has exposed major evidence gaps for the treatment of these disorders and incentivizes large pediatric trials, particularly on treatment options for which, to date, no evidence exists.
Keywords: disorders of gut brain interaction; functional abdominal pain; gut‐brain psychotherapies; irritable bowel syndrome; pediatric.
© 2025 The Author(s). Journal of Pediatric Gastroenterology and Nutrition published by Wiley Periodicals LLC on behalf of European Society for Pediatric Gastroenterology, Hepatology, and Nutrition and North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition.
Conflict of interest statement
Regulations for conflict of interest followed the Cochrane handbook as well as the GRADE body. These implied that not only the receipt of financial contributions of institutions with links to assessed treatment options implied a conflict, but academic involvement in research on a specific treatment option without commercial funding as well. Marc Alexander Benninga indicated conflicts of interest for Cognitive Behavioral Therapy (CBT), Yoga, Hypnotherapy, and laxatives, due to involvement in studies assessing these therapies. Marc Alexander Benninga also indicated consulting services for Norgine, Coloplast, Wellspect, Allergan, Mallinckrodt, United Pharmaceuticals, Danone, FrieslandCampina, and HIPP. Marc Alexander Benninga also served as speaker for Abbott and Menarini. Ashish Chogle indicated a conflict of interest for neurostimulation, due to involvement in studies assessing these therapies. Julie Khlevner indicated a conflict of interest for laxatives, due to involvement in studies assessing these therapies. Julie Khlevner also indicated consulting services for Abbvie Inc. Carlo Di Lorenzo indicated conflicts of interest for neurostimulation, amitriptyline, linaclotide, and prucalopride, due to involvement in studies assessing these therapies. Miguel Saps indicated conflicts of interest for amitriptyline and linaclotide, due to involvement in studies assessing these therapies. Miguel Saps also indicated consulting services for Abbvie Inc., IQVIA, and Focus Medical Communications. Nikhil Thapar indicated conflicts of interest for probiotics, due to receiving honoraria from both Biogaia and Nutricia for roles in the advisory board and in moderating a key opinion leaders meeting on the addition of pre‐/probiotics in infant formula, respectively. Arine Vlieger indicated a conflict of interest for Hypnotherapy, due to involvement in studies assessing these therapies. Arine Vlieger also indicated an unpaid position as Chair of the Dutch Society for Hypnosis in Children. The remaining authors declare no conflicts of interest.
Figures
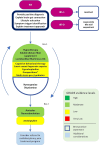
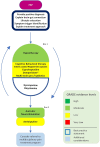

References
-
- Youssef NN, Atienza K, Langseder AL, Strauss RS. Chronic abdominal pain and depressive symptoms: analysis of the national longitudinal study of adolescent health. Clin Gastroenterol Hepatol. 2008;6(3):329‐332. - PubMed
-
- Hoekman DR, Rutten JMTM, Vlieger AM, Benninga MA, Dijkgraaf MGW. Annual costs of care for pediatric irritable bowel syndrome, functional abdominal pain, and functional abdominal pain syndrome. J Pediatr. 2015;167(5):1103‐1108.e2. - PubMed
-
- Lewis ML, Palsson OS, Whitehead WE, van Tilburg MAL. Prevalence of functional gastrointestinal disorders in children and adolescents. J Pediatr. 2016;177:39‐43.e3. - PubMed
-
- Assa A, Ish‐Tov A, Rinawi F, Shamir R. School attendance in children with functional abdominal pain and inflammatory bowel diseases. J Pediatr Gastroenterol Nutr. 2015;61(5):553‐557. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

