Encorafenib, Cetuximab, and mFOLFOX6 in BRAF-Mutated Colorectal Cancer
- PMID: 40444708
- PMCID: PMC12197837
- DOI: 10.1056/NEJMoa2501912
Encorafenib, Cetuximab, and mFOLFOX6 in BRAF-Mutated Colorectal Cancer
Abstract
Background: First-line treatment with encorafenib plus cetuximab (EC) with or without chemotherapy (oxaliplatin, leucovorin, and fluorouracil [mFOLFOX6]) for BRAF V600E-mutated metastatic colorectal cancer, an aggressive subtype with a poor prognosis, was compared with standard care (chemotherapy with or without bevacizumab) in an open-label, phase 3 trial, which showed significance regarding one of the two primary end points, objective response according to blinded independent central review (odds ratio for EC+mFOLFOX6 vs. standard care, 2.44; one-sided P<0.001). This result led to accelerated Food and Drug Administration approval of this investigational combination therapy for BRAF V600E-mutated metastatic colorectal cancer, including as first-line therapy. Data on progression-free survival (the second primary end point) and an updated interim analysis of overall survival are now available.
Methods: We randomly assigned patients with untreated BRAF V600E-mutated metastatic colorectal cancer to receive EC, EC+mFOLFOX6, or standard care. The two primary end points were objective response (reported previously) and progression-free survival according to blinded independent central review in the EC+mFOLFOX6 group and the standard-care group. The key secondary end point was overall survival.
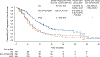
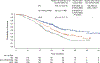
Results: Significantly longer progression-free survival was seen with EC+mFOLFOX6 than with standard care (median, 12.8 vs. 7.1 months; hazard ratio for progression or death, 0.53; 95% confidence interval [CI], 0.41 to 0.68; P<0.001). In an interim analysis, overall survival was significantly longer with EC+mFOLFOX6 than with standard care (median, 30.3 vs. 15.1 months; hazard ratio for death, 0.49; 95% CI, 0.38 to 0.63; P<0.001). The incidence of serious adverse events during treatment was 46.1% with EC+mFOLFOX6 and 38.9% with standard care. Safety profiles were consistent with those known for each agent.
Conclusions: This trial showed significantly longer progression-free survival and overall survival with first-line treatment with EC+mFOLFOX6 than with standard care among patients with BRAF V600E-mutated metastatic colorectal cancer. (Funded by Pfizer and others; BREAKWATER ClinicalTrials.gov number, NCT04607421.).
Copyright © 2025 Massachusetts Medical Society.
Figures


References
-
- Tabernero J, Ros J, Élez E. The evolving treatment landscape in BRAF-V600E–mutated metastatic colorectal cancer. Am Soc Clin Oncol Educ Book 2022;42:254–63. - PubMed
-
- Delord JP, Robert C, Nyakas M, et al. Phase I dose-escalation and -expansion study of the BRAF inhibitor encorafenib (LGX818) in metastatic BRAF-mutant melanoma. Clin Cancer Res 2017;23:5339–48. - PubMed
-
- Stuart DD, Li N, Poon DJ, et al. Abstract 3790: Preclinical profile of LGX818: a potent and selective RAF kinase inhibitor. Cancer Res 2012;72(8_Suppl):3790.
Publication types
MeSH terms
Substances
Supplementary concepts
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
