Providing 2 Types of mHealth Interventions to Support Self-Management Among People Living With HIV: Randomized Clinical Trial
- PMID: 40444823
- PMCID: PMC12140505
- DOI: 10.2196/60905
Providing 2 Types of mHealth Interventions to Support Self-Management Among People Living With HIV: Randomized Clinical Trial
Abstract
Background: Mobile health (mHealth) has been continuously developed to support the HIV care continuum for people living with HIV. Considering the practical needs and acceptability of digital health solutions, it is essential to explore effective content and diverse delivery methods for self-management support.
Objective: This study aimed to assess the effectiveness of 2 non-face-to-face mHealth interventions for people living with HIV. We compared the impact on HIV self-management of (1) a link group, which received access to information via mobile link, and (2) an app group, which used a mobile app enabling information exploration and self-recording of health outcomes, including medication adherence, symptoms, mental health score, and sexual safety.
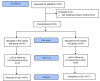
Methods: A 2-arm, prospective, randomized clinical trial was conducted, involving 83 people living with HIV aged 19 years or older, who were assigned to the app group (n=42) or link group (n=41). The primary outcome was self-reported self-efficacy for HIV management (HIV-SE), which comprised 6 domains: managing depression or mood, medication, symptoms, and fatigue; communicating with health care providers; and getting support or help. A paired t test and generalized estimating equation were used to analyze the outcomes at baseline, 4 weeks postintervention, and 8 weeks after an additional 4-week voluntary use period.
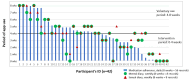
Results: Both groups demonstrated improvements in total HIV-SE scores at 4 weeks compared with baseline. All domain scores improved in the app group, with a significant increase in total HIV-SE and managing fatigue. The link group significantly improved in managing depression or mood, fatigue, and getting support or help domains. The generalized estimating equation analysis indicated that, compared with the link group, the app group had significant group-by-time interaction with a positive effect on managing symptoms at 4 weeks (β=0.635, 95% CI 0.023 to 1.247; P=.04) but a negative effect on managing depression or mood at 8 weeks (β=-0.824, 95% CI -1.448 to -0.200; P=.01). Only 9.5% (4/42) of app group participants maintained daily visits during the voluntary use period of 4 to 8 weeks.
Conclusions: Both types of informational mHealth interventions, through mobile apps or link access, contributed to improving HIV-SE. Delivering information via direct text message links could be suitable for individuals who are hesitant to use HIV-related apps. While mobile apps promote self-monitoring and symptom management through self-recording and reflection, strategies are needed to sustain long-term app engagement. In addition, user-customized psychiatric content beyond mental health recordings has been suggested for managing depressed moods in mHealth interventions.
Keywords: HIV; mHealth; mobile health; randomized clinical trial; self-management; telemedicine.
© Gwang Suk Kim, Layoung Kim, Seoyoung Baek, Sooyoung Kwon, Ji Min Kim, Jun Yong Choi, Jae-Phil Choi. Originally published in JMIR mHealth and uHealth (https://mhealth.jmir.org).
Conflict of interest statement
Figures

Similar articles
-
Attitudes, Beliefs, and Willingness Toward the Use of mHealth Tools for Medication Adherence in the Florida mHealth Adherence Project for People Living With HIV (FL-mAPP): Pilot Questionnaire Study.JMIR Mhealth Uhealth. 2019 Jul 3;7(7):e12900. doi: 10.2196/12900. JMIR Mhealth Uhealth. 2019. PMID: 31271150 Free PMC article.
-
Association Between User Engagement of a Mobile Health App for Gout and Improvements in Self-Care Behaviors: Randomized Controlled Trial.JMIR Mhealth Uhealth. 2019 Aug 13;7(8):e15021. doi: 10.2196/15021. JMIR Mhealth Uhealth. 2019. PMID: 31411147 Free PMC article. Clinical Trial.
-
Evaluating the Effectiveness of a Mobile App for Breast Cancer Self-Management on Self-Efficacy: Nonrandomized Intervention Trial.JMIR Mhealth Uhealth. 2025 Mar 26;13:e63989. doi: 10.2196/63989. JMIR Mhealth Uhealth. 2025. PMID: 40138696 Free PMC article.
-
Mobile Apps for Common Noncommunicable Disease Management: Systematic Search in App Stores and Evaluation Using the Mobile App Rating Scale.JMIR Mhealth Uhealth. 2024 Mar 12;12:e49055. doi: 10.2196/49055. JMIR Mhealth Uhealth. 2024. PMID: 38532298 Free PMC article.
-
Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.Cochrane Database Syst Rev. 2022 Feb 1;2(2022):CD014217. doi: 10.1002/14651858.CD014217. Cochrane Database Syst Rev. 2022. PMID: 36321557 Free PMC article.
References
-
- Oh KS, Lee J soo, Han E. Risk factors associated with medication adherence in HIV/AIDS patients. Korean J Clin Pharm. 2023 Dec 31;33(4):254–260. doi: 10.24304/kjcp.2023.33.4.254. doi. - DOI
-
- HIV/AIDS notifications in the Republic of Korea. Korea Disease Control and Prevention Agency. 2021. [15-01-2024]. www.kdca.go.kr/contents.es?mid=a20301070504 URL. Accessed.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

