Administration of 2-deoxy-D-glucose alleviates cancer-induced bone pain by suppressing microglial polarization to the M1 phenotype and neuroinflammation
- PMID: 40444883
- PMCID: PMC12171252
- DOI: 10.1177/17448069251348778
Administration of 2-deoxy-D-glucose alleviates cancer-induced bone pain by suppressing microglial polarization to the M1 phenotype and neuroinflammation
Abstract
Background: Cancer-induced bone pain (CIBP) is a debilitating complication with few effective treatments. Microglial activation contributes to the progression of CIBP. 2-deoxy-D-glucose (2-DG), a glycolytic inhibitor, could inhibit microglial activation. Its effect on CIBP remains unclear. This study aims to investigate the role of 2-DG in CIBP mice and underlying mechanisms.
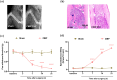
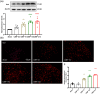
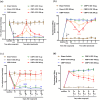
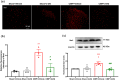
Methods: In this research, we established a CIBP mouse model by injecting Lewis lung cancer (LLC) cells into the bone marrow of the femur. Relevant pain behaviors were assessed by measuring the paw withdrawal threshold and spontaneous hind limb lifting. Additionally, the glycolysis inhibitor 2-DG was intrathecally administered to treat CIBP in mice. Western blotting and immunofluorescence techniques were employed to analyze microglial activation and M1/M2 phenotype markers in the spinal cord.
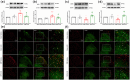
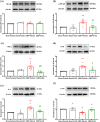
Results: Our findings demonstrated significant microglial activation and polarization toward the M1 phenotype in the spinal cord of CIBP mice. Intrathecal administration of 2-DG effectively alleviated pain-related behaviors in CIBP mice. Furthermore, this treatment suppressed microglial activation and M1 polarization, while significantly restoring levels of the M2 phenotype. Additionally, 2-DG attenuated the production of pro-inflammatory factors such as tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), and interleukin-6 (IL-6), while boosting the secretion of the anti-inflammatory factor (IL-10) in the spinal cord of CIBP mice. Notably, 2-DG effectively suppresses microglia activation and M1 polarization in LPS + IFN-γ-induced BV-2 cells by downregulating CD86, iNOS expression, TNF-α, IL-1β, IL-6 levels while upregulating Arg-1, CD206 expression and IL-10 level.
Conclusion: These results suggest that 2-DG ameliorates mechanical allodynia, spontaneous pain and neuroinflammation in the spinal cord of CIBP mice by promoting the transition from the M1 phenotype to the M2 phenotype. This study may provide a novel strategy for the treatment of CIBP.
Keywords: 2-deoxy-D-glucose; Cancer-induced bone pain; microglia polarization; neuroinflammation.
Conflict of interest statement
Declaration of conflicting interestsThe author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures



References
-
- Coleman RE. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin Cancer Res 2006; 12: 6243s–6249s. - PubMed
-
- Tu H, Chu H, Guan S, Hao F, Xu N, Zhao Z, Liang Y. The role of the M1/M2 microglia in the process from cancer pain to morphine tolerance. Tissue Cell 2021; 68: 101438. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

