Defining neuronal responses to the neurotropic parasite Toxoplasma gondii
- PMID: 40444956
- PMCID: PMC12188731
- DOI: 10.1128/msphere.00216-25
Defining neuronal responses to the neurotropic parasite Toxoplasma gondii
Abstract
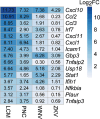
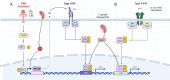
A select group of pathogens infects neurons in the brain. Prior dogma held that neurons were "defenseless" against infecting microbes, but many studies suggest that neurons can mount anti-microbial defenses. However, a knowledge gap in understanding how neurons respond in vitro and in vivo to different classes of microorganisms remains. To address this gap, we compared a transcriptional data set derived from primary neuron cultures (PNCs) infected with the neurotropic intracellular parasite Toxoplasma gondii with a data set derived from neurons injected with T. gondii protein in vivo. These curated responses were then compared to the transcriptional responses of PNCs infected with the single-stranded RNA viruses, West Nile virus or Zika virus. These analyses highlighted a conserved response to infection associated with chemokines (Cxcl10, Ccl2) and cytokines (interferon signaling). However, T. gondii had diminished IFN-α signaling in vitro compared to the viral data sets and was uniquely associated with a decrease in neuron-specific genes (Snap25, Slc17a7, Prkcg). These data underscore that neurons participate in infection-induced neuroinflammation and illustrate that neurons possess both pathogen-specific and pathogen-conserved responses.IMPORTANCEThough neurons are commonly the target of pathogens that infect the central nervous system (CNS), few data sets assess the neuronal response to infection. This paucity of data is likely because neurons are perceived to have diminished immune capabilities. However, to understand the role of neurons in neuroinflammation and their immune capabilities, their responses must be investigated. Here, we analyzed publicly accessible, neuron-specific data sets to compare neuron responses to a eukaryotic pathogen vs two Orthoflaviviruses. A better understanding of neuron responses to different infections will allow us to develop methods for inhibiting pathways that lead to neuron dysfunction, enhancing those that limit pathogen survival, and mitigating infection-induced damage to the CNS.
Keywords: RNA-seq; T. gondii; Toxoplasma gondii; central nervous system infections; host response; neurons; transcriptomics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Update of
-
Defining neuronal responses to the neurotropic parasite Toxoplasma gondii.bioRxiv [Preprint]. 2025 Mar 31:2025.03.31.645603. doi: 10.1101/2025.03.31.645603. bioRxiv. 2025. Update in: mSphere. 2025 Jun 25;10(6):e0021625. doi: 10.1128/msphere.00216-25. PMID: 40236177 Free PMC article. Updated. Preprint.
Comment in
-
To be or not to be: how does the brain respond to different infectious agents?mSphere. 2025 Aug 26;10(8):e0032625. doi: 10.1128/msphere.00326-25. Epub 2025 Aug 5. mSphere. 2025. PMID: 40762468 Free PMC article.
References
-
- Garen PD, Tsai TF, Powers JM. 1999. Human eastern equine encephalitis: immunohistochemistry and ultrastructure. Mod Pathol 12:646–652. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
