A novel silver-ruthenium-based antimicrobial kills Gram-negative bacteria through oxidative stress-induced macromolecular damage
- PMID: 40444966
- PMCID: PMC12188735
- DOI: 10.1128/msphere.00017-25
A novel silver-ruthenium-based antimicrobial kills Gram-negative bacteria through oxidative stress-induced macromolecular damage
Abstract
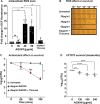
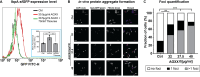
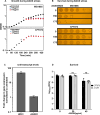
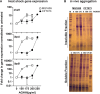
Amplified by the decline in antibiotic discovery, the rise of antibiotic resistance has become a significant global challenge in infectious disease control. Extraintestinal Escherichia coli (ExPEC), known to be the most common instigators of urinary tract infections (UTIs), represents such a global threat. Novel strategies for more efficient treatments are therefore desperately needed. These include silver nanoparticles, which have been used as antimicrobial surface coatings on catheters to eliminate biofilm-forming uropathogens and reduce the risk of nosocomial infections. AGXX is a promising silver-ruthenium coating that presumably kills bacteria through the generation of reactive oxygen species (ROS). However, neither AGXX's mode of action is fully understood, nor have its effects on Gram-negative bacteria or bacterial response and defense mechanisms toward AGXX been studied in detail. Here, we report that the bactericidal effects of AGXX are primarily based on ROS formation, as supplementation of the media with a ROS scavenger completely abolished AGXX-induced killing. We further show that AGXX impairs the integrity of the bacterial cell envelope and causes substantial protein aggregation and DNA damage already at sublethal concentrations. ExPEC strains appear to be more resistant to the proteotoxic effects of AGXX compared to non-pathogenic E. coli, indicating improved defense capabilities of the uropathogen. Global transcriptomic studies of AGXX-stressed ExPEC revealed a strong oxidative stress response, perturbations in metal homeostasis, as well as the activation of heat shock and DNA damage responses. Finally, we present evidence that ExPEC counteracts AGXX damage through the production of the chaperone polyphosphate, protecting cells from protein aggregation.IMPORTANCEThe rise in drug-resistant bacteria, together with the decline in antibiotic development, requires new strategies for infectious disease control. Gram-negative pathogens are particularly challenging to combat due to their outer membrane. This study highlights the effectiveness of the silver-containing antimicrobial AGXX against the Gram-negative bacterium Escherichia coli. AGXX effectively reduces bacterial survival by interfering with the membrane integrity and causing DNA damage and protein aggregation, which is likely a consequence of uncontrolled generation of oxidative stress. Our findings emphasize AGXX's potential as an antimicrobial surface coating and shed light on potential targets to reduce bacterial resistance to AGXX.
Keywords: antimicrobial agents; oxidative damage; oxidative stress; polyphosphate; silver; stress response.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Update of
-
A Novel Silver-Ruthenium-Based Antimicrobial Kills Gram-Negative Bacteria Through Oxidative Stress-Induced Macromolecular Damage.bioRxiv [Preprint]. 2025 Jan 4:2025.01.03.631245. doi: 10.1101/2025.01.03.631245. bioRxiv. 2025. Update in: mSphere. 2025 Jun 25;10(6):e0001725. doi: 10.1128/msphere.00017-25. PMID: 39803548 Free PMC article. Updated. Preprint.
References
-
- Vihta K-D, Stoesser N, Llewelyn MJ, Quan TP, Davies T, Fawcett NJ, Dunn L, Jeffery K, Butler CC, Hayward G, Andersson M, Morgan M, Oakley S, Mason A, Hopkins S, Wyllie DH, Crook DW, Wilcox MH, Johnson AP, Peto TEA, Walker AS. 2018. Trends over time in Escherichia coli bloodstream infections, urinary tract infections, and antibiotic susceptibilities in Oxfordshire, UK, 1998-2016: a study of electronic health records. Lancet Infect Dis 18:1138–1149. doi: 10.1016/S1473-3099(18)30353-0 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
