Chemoautotrophy in subzero environments and the potential for cold-adapted Rubisco
- PMID: 40444981
- PMCID: PMC12175532
- DOI: 10.1128/aem.00604-25
Chemoautotrophy in subzero environments and the potential for cold-adapted Rubisco
Abstract
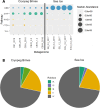
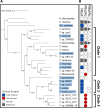
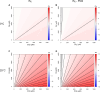
The act of fixing inorganic carbon into the biosphere is largely facilitated by one enzyme, Rubisco. Beyond well-studied plants and cyanobacteria, many bacteria use Rubisco for chemolithoautotrophy in extreme environments on Earth. Here, we characterized the diversity of autotrophic pathways and chemolithoautotrophic Rubiscos from two distinct subzero, hypersaline Arctic environments: 40-kyr relic marine brines encased within permafrost (cryopeg brines) and first-year sea ice. The Calvin-Benson-Bassham (CBB) cycle was widely found in both environments, although with different predominant Rubisco forms. From cryopeg brine, reconstructions of metagenome-assembled genomes (MAGs) uncovered four MAGs with the potential for chemolithoautotrophy, of which the CBB-containing genus Thiomicrorhabdus was most abundant. A broader survey of Thiomicrorhabdus genomes from diverse environments identified a core complement of three Rubisco forms (II, IAc, IAq) with a complex pattern of gain and loss, with form II constitutively present in genomes from subzero environments. Using representative kinetic data, we modeled carboxylation rates of Rubisco forms II, IAc, and IAq across CO2, O2, and temperature conditions. We found that form II outcompetes form I at low O2, but cold temperatures minimize this advantage. Inspection of form II from genomes from cold environments identified signals of potential thermal adaptation due to key amino acid substitutions, which resulted in a more exposed active site. We argue that subzero form II from Thiomicrorhabdus warrants further study as it may have unique kinetics or thermal stability. This work can help address the limits of autotrophic functionality in extreme environments on Earth and other planetary bodies.IMPORTANCEAutotrophy, or the fixation of inorganic carbon to biomass, is a key factor in life's ability to thrive on Earth. Research on autotrophy has focused on plants and algae, but many bacteria are also autotrophic and can survive and thrive under more extreme conditions. These bacteria are a window to past autotrophy on Earth, as well as potential autotrophy in extreme environments elsewhere in the universe. Our study focused on dark, cold, saline environments, which are likely to be found on Enceladus and Europa, as well as in the Martian subsurface. We found evidence for potential cold adaptation in a key autotrophic enzyme, Rubisco, which could expand the known boundaries of autotrophy in rapidly disappearing icy environments on Earth. We also present a novel model framework that can be used to probe the limits of autotrophy not only on Earth but also on key astrobiological targets like Enceladus and Europa.
Keywords: Rubisco; astrobiology; autotrophs; chemoautotrophy; psychrophiles.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

