Evaluation of Different Cryoprotectant Combinations in Testicular Vitrification in Dogs
- PMID: 40445065
- PMCID: PMC12124239
- DOI: 10.1111/rda.70081
Evaluation of Different Cryoprotectant Combinations in Testicular Vitrification in Dogs
Abstract
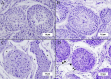
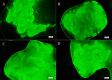
Testicular vitrification requires the use of high concentrations of cryoprotectants, which can cause damage to samples due to their toxicity. The combination of these substances comes up as a way to mitigate this problem. Thus, the aim of this study was to evaluate three cryoprotectant combinations in the testicular vitrification of dogs. Ten testicular pairs from adult dogs were used, from which 12 fragments of each pair were obtained, distributed among the fresh control group (CTR) and the experimental groups according to the cryoprotectant combinations tested: dimethyl sulfoxide (DMSO)/ethylene glycol (EG), DMSO/glycerol (GLY), and EG/GLY. The fragments were vitrified using the solid surface vitrification method (SSV), at a final concentration of 5.6 mol/L of the combined cryoprotectants. Subsequently, they were warmed up and processed for histomorphological morphometric evaluations and determination of mitochondrial activity with Rhodamine 123. Considering the morphological evaluation, the DMSO/EG group showed results similar to CTR, with good scores for nuclear integrity and cell organisation in the seminiferous tubules (p > 0.05). In contrast, the EG/GLY group presented greater nuclear condensation. It was difficult to visualise and distinguish between spermatogonia and Sertoli cells (p < 0.05). The DMSO/GLY group also showed distinct levels between spermatogonia and Sertoli cells, as well as nuclear condensation, which statistically differed from CTR (p < 0.05). Also, it was observed a random distribution of the remaining cells in the seminiferous tubules of the EG/GLY and DMSO/GLY groups. The three tested groups showed basement membrane retraction and a reduction of approximately 11.6% in the average diameter of the seminiferous tubules (p < 0.05). Vitrification did not influence the mitochondrial activity of the samples, regardless of the combination of cryoprotectants used (p > 0.05). It was concluded that the DMSO/EG combination best contributed to the maintenance of the testicular histomorphological structure of dogs after vitrification.
Keywords: cryopreservation; dimethyl sulfoxide; ethylene glycol; glycerol; testicles.
© 2025 The Author(s). Reproduction in Domestic Animals published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


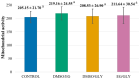
References
-
- Andrae, C. S. , Oliveira E. C. S., Ferraz M. A. M. M., and Nagashima J. B.. 2021. “Cryopreservation of Grey Wolf (<styled-content style="fixed-case"> Canis lupus </styled-content>) Testicular Tissue.” Cryobiology 100: 173–179. - PubMed
-
- Baert, Y. , Goossens E., Saen D. V., Ning L., Veld P., and Tournaye H.. 2012. “Orthotopic Grafting of Cryopreserved Prepubertal Testicular Tissue: In Search of a Simple Yet Effective Cryopreservation Protocol.” Fertility and Sterility 97, no. 5: 1152–1157. 10.1016/j.fertnstert.2012.02.010. - DOI - PubMed
-
- Buarpung, S. , Tharasanit T., Comizzoli P., and Techakumphu M.. 2013. “Feline Spermatozoa From Fresh and Cryopreserved Testicular Tissues Have Comparable Ability to Fertilize Matured Oocytes and Sustain the Embryo Development After Intracytoplasmic Sperm Injection.” Theriogenology 79, no. 1: 149–158. 10.1016/j.theriogenology.2012.09.022. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

