Interactive Explainable Deep Learning Model for Hepatocellular Carcinoma Diagnosis at Gadoxetic Acid-enhanced MRI: A Retrospective, Multicenter, Diagnostic Study
- PMID: 40445095
- PMCID: PMC12130696
- DOI: 10.1148/rycan.240332
Interactive Explainable Deep Learning Model for Hepatocellular Carcinoma Diagnosis at Gadoxetic Acid-enhanced MRI: A Retrospective, Multicenter, Diagnostic Study
Abstract
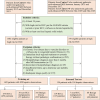
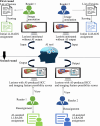
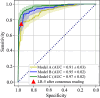
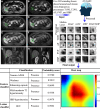
Purpose To develop an artificial intelligence (AI) model based on gadoxetic acid-enhanced MRI to assist radiologists in hepatocellular carcinoma (HCC) diagnosis. Materials and Methods This retrospective study included patients with focal liver lesions (FLLs) who underwent gadoxetic acid-enhanced MRI between January 2015 and December 2021. All hepatic malignancies were diagnosed pathologically, whereas benign lesions were confirmed with pathologic findings or imaging follow-up. Five manually labeled bounding boxes for each FLL obtained from precontrast T1-weighted, T2-weighted, arterial phase, portal venous phase, and hepatobiliary phase images were included. The lesion classifier component, used to distinguish HCC from non-HCC, was trained and externally tested. The feature classifier, based on a post hoc algorithm, inferred the presence of the Liver Imaging Reporting and Data System (LI-RADS) features by analyzing activation patterns of the pretrained lesion classifier. Two radiologists categorized FLLs in the external testing dataset according to LI-RADS criteria. Diagnostic performance of the AI model and the model's impact on reader accuracy were assessed. Results The study included 839 patients (mean age, 51 years ± 12 [SD]; 681 male) with 1023 FLLs (594 HCCs and 429 non-HCCs). The AI model yielded area under the receiver operating characteristic curves of 0.98 and 0.97 in the training set and external testing set, respectively. Compared with LI-RADS category 5, the AI model showed higher sensitivity (91.6% vs 74.8%; P < .001) and similar specificity (90.7% vs 96.0%; P = .22). The two readers identified more LI-RADS major features and more accurately classified category LR-5 lesions when assisted versus unassisted by AI, with higher sensitivities (reader 1, 85.7% vs 72.3%; P < .001; reader 2, 89.1% vs 74.0%; P < .001) and the same specificities (reader 1, 93.3% vs reader 2, 94.7%; P > .99 for both). Conclusion The AI model accurately diagnosed HCC and improved the radiologists' diagnostic performance. Keywords: Artificial Intelligence, Deep Learning, MRI, Hepatocellular Carcinoma Supplemental material is available for this article. © RSNA, 2025 See also commentary by Singh et al in this issue.
Keywords: Artificial Intelligence; Deep Learning; Hepatocellular Carcinoma; MRI.
Conflict of interest statement
Figures



Similar articles
-
Impact of LI-RADS CT and MRI Ancillary Features on Diagnostic Performance: An Individual Participant Data Meta-Analysis.Radiology. 2025 Jul;316(1):e242278. doi: 10.1148/radiol.242278. Radiology. 2025. PMID: 40626878
-
Based on Gadolinium Ethoxybenzyl DTPA-Enhanced MRI: Diagnostic Performance of the Category-Modified LR-5 Criteria in Patients At Risk for Hepatocellular Carcinoma.Technol Cancer Res Treat. 2024 Jan-Dec;23:15330338241256859. doi: 10.1177/15330338241256859. Technol Cancer Res Treat. 2024. PMID: 38780516 Free PMC article.
-
Contrast-enhanced ultrasound-based AI model for multi-classification of focal liver lesions.J Hepatol. 2025 Aug;83(2):426-439. doi: 10.1016/j.jhep.2025.01.011. Epub 2025 Jan 21. J Hepatol. 2025. PMID: 39848548
-
Do transition and hepatobiliary phase hypointensity improve LI-RADS categorization as an alternative washout: a systematic review and meta-analysis.Eur Radiol. 2022 Aug;32(8):5134-5143. doi: 10.1007/s00330-022-08665-y. Epub 2022 Mar 10. Eur Radiol. 2022. PMID: 35267090
-
Contrast-enhanced ultrasound for the diagnosis of hepatocellular carcinoma in adults with chronic liver disease.Cochrane Database Syst Rev. 2022 Sep 2;9(9):CD013483. doi: 10.1002/14651858.CD013483.pub2. Cochrane Database Syst Rev. 2022. PMID: 36053210 Free PMC article.
Cited by
-
Beyond the Black Box: Explainable AI Transforms Hepatocellular Carcinoma Diagnosis at MRI.Radiol Imaging Cancer. 2025 May;7(3):e250198. doi: 10.1148/rycan.250198. Radiol Imaging Cancer. 2025. PMID: 40445096 Free PMC article. No abstract available.
References
-
- Sung H , Ferlay J , Siegel RL , et al. . Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries . CA Cancer J Clin 2021. ; 71 ( 3 ): 209 – 249 . - PubMed
-
- Vogel A , Meyer T , Sapisochin G , Salem R , Saborowski A . Hepatocellular carcinoma . Lancet 2022. ; 400 ( 10360 ): 1345 – 1362 . - PubMed
-
- Kim SH , Kim SH , Lee J , et al. . Gadoxetic acid-enhanced MRI versus triple-phase MDCT for the preoperative detection of hepatocellular carcinoma . AJR Am J Roentgenol 2009. ; 192 ( 6 ): 1675 – 1681 . - PubMed
-
- Sano K , Ichikawa T , Motosugi U , et al. . Imaging study of early hepatocellular carcinoma: usefulness of gadoxetic acid-enhanced MR imaging . Radiology 2011. ; 261 ( 3 ): 834 – 844 . - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

