A suite of selective pressures supports the maintenance of alleles of a Drosophila immune peptide
- PMID: 40445192
- PMCID: PMC12124834
- DOI: 10.7554/eLife.90638
A suite of selective pressures supports the maintenance of alleles of a Drosophila immune peptide
Abstract
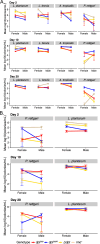
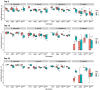
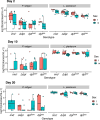
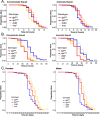
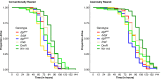
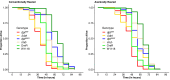
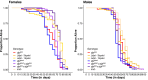
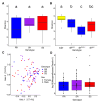
The innate immune system provides hosts with a crucial first line of defense against pathogens. While immune genes are often among the fastest evolving genes in the genome, in Drosophila, antimicrobial peptides (AMPs) are notable exceptions. Instead, AMPs may be under balancing selection, such that over evolutionary timescales, multiple alleles are maintained in populations. In this study, we focus on the Drosophila AMP Diptericin A, which has a segregating amino acid polymorphism associated with differential survival after infection with the Gram-negative bacteria Providencia rettgeri. Diptericin A also helps control opportunistic gut infections by common Drosophila gut microbes, especially those of Lactobacillus plantarum. In addition to genotypic effects on gut immunity, we also see strong sex-specific effects that are most prominent in flies without functional diptericin A. To further characterize differences in microbiomes between different diptericin genotypes, we used 16S metagenomics to look at the microbiome composition. We used both lab-reared and wild-caught flies for our sequencing and looked at overall composition as well as the differential abundance of individual bacterial families. Overall, we find flies that are homozygous for one allele of diptericin A are better equipped to survive a systemic infection from P. rettgeri, but in general have a shorter lifespans after being fed common gut commensals. Our results suggest a possible mechanism for the maintenance of genetic variation of diptericin A through the complex interactions of sex, systemic immunity, and the maintenance of the gut microbiome.
Keywords: D. melanogaster; Diptericin; Providencia; antimicrobial peptide; evolutionary biology; infectious disease; innate immunity; microbiology.
© 2023, Mullinax et al.
Conflict of interest statement
SM, AD, AG, PC, BS, RU No competing interests declared
Figures

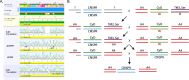
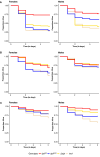

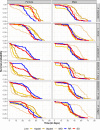
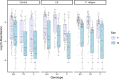
Update of
-
A suite of selective pressures supports the maintenance of alleles of a Drosophila immune peptide.bioRxiv [Preprint]. 2024 Jul 29:2023.08.18.553899. doi: 10.1101/2023.08.18.553899. bioRxiv. 2024. Update in: Elife. 2025 May 30;12:RP90638. doi: 10.7554/eLife.90638. PMID: 37662279 Free PMC article. Updated. Preprint.
References
-
- Badinloo M, Nguyen E, Suh W, Alzahrani F, Castellanos J, Klichko VI, Orr WC, Radyuk SN. Overexpression of antimicrobial peptides contributes to aging through cytotoxic effects in Drosophila tissues. Archives of Insect Biochemistry and Physiology. 2018;98:e21464. doi: 10.1002/arch.21464. - DOI - PMC - PubMed
-
- Bagnicka E, Strzałkowska N, Jóźwik A, Krzyżewski J, Horbańczuk J, Zwierzchowski L. Expression and polymorphism of defensins in farm animals. Acta Biochimica Polonica. 2010;57:487–497. - PubMed
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

