Biphasic contrast-enhanced [18F]PSMA-1007 PET/CT imaging to improve the detection of local relapse of prostate cancer
- PMID: 40445472
- PMCID: PMC12125407
- DOI: 10.1186/s13550-025-01252-4
Biphasic contrast-enhanced [18F]PSMA-1007 PET/CT imaging to improve the detection of local relapse of prostate cancer
Abstract
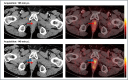
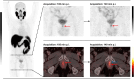
Background: The implementation of PSMA imaging in prostate cancer (PC) management has significantly improved the medical care of patients owing to its clinical impact, particularly with respect to biochemical recurrence. However, there is still an unmet clinical need regarding the correct discrimination of equivocal, centrally located, focal [18F]PSMA-1007 uptake without any CT-morphological findings in the postsurgical prostate bed. The aim of this monocentric, retrospective study was to investigate the efficacy of a biphasic, contrast-enhanced [18F]PSMA-1007 acquisition protocol.
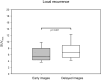
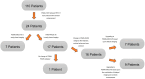
Results: This study investigated a total of 24 biologically male patients with BCR, with a mean PSA level of 0.96 ng/ml at the time of recurrence. The presence of local relapse was regarded as consistent by biphasic, contrast-enhanced [18F]PSMA-1007 PET/CT scans, of which 22 cases were finally validated through the composite reference standard after a 2-years follow-up. The acquisition of whole-body, contrast-enhanced PET/CT imaging data was performed after a mean of 105 (± 19) minutes, whereas late-phase PET/CT imaging of the pelvis with low-dose CT was conducted after 140 min (± 10) on average following the intravenous application of [18F]PSMA-1007 (injected mean activity of 240 MBq (± 29)). The median SUVmax and SUVmean values of local relapse increased by 26% and 5%, respectively, in late-phase images. Moreover, median TBR with respect to the obturator internus muscle seemed to benefit the most from late-phase imaging, with an increase of 185%. The dynamics of the SUV metrics and TBR in lesions were statistically significant (P value < 0.001-0.019). Moreover, the retrospective reading of delayed [18F]PSMA-1007 PET/CT imaging provided an upgrade of the reporting for suspected local PC relapse from a previous PSMA-RADS 3A to a later PSMA-RADS 5 in seven patients (29%), unless the impact of contrast agent in the urethra would also be considered equally important. For the remaining patients, the qualitative evaluation of contrast agent displacement in the urethra was necessary for a final clinical decision that provided the upgrading of the reporting to PSMA RADS 5 for an additional nine patients (38%).
Conclusions: Given the aforementioned, highly specific unmet clinical need for a relatively small ratio of patients with prostate cancer undergoing PSMA imaging, our proposed acquisition protocol mandates a well-balanced preselection of patients. Under this premise, the study results demonstrated that the optimized acquisition protocol with biphasic contrast-enhanced [18F]PSMA-1007 PET/CT imaging improved the diagnostic performance for the detection of local PC recurrence in 67% of preselected patients.
Keywords: Biphasic PSMA imaging; Contrast enhancement; Late imaging; Local relapse; PSMA imaging; [18F]PSMA-1007.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval: All procedures performed in studies involving human participants were approved by the ethics committee and carried out in accordance with the ethical standards of the institutional and/or national research committees and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The study received approval from the Ethical Committee of the Medical Faculty of Heinrich-Heine University Duesseldorf, Germany (Study-Nr.: 2022–1898). Clinical trial number: not applicable. Consent to participate: Informed consent for participation was obtained from all individual participants included in the study, and the investigation was conducted according to national regulatory laws. Consent for publication: Not applicable. Competing interests: FLG has patent applications for [18F]PSMA-1007 and is an advisor at ABX, Telix, and SOFIE Biosciences. The authors have no relevant financial or nonfinancial interests to disclose.
Figures
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

