Electrodeposited ZnO/Zn(OH)2 Nanosheets as a Functional Interface for Dendrite-Free Lithium Metal Anodes
- PMID: 40445614
- PMCID: PMC12332819
- DOI: 10.1002/smll.202503607
Electrodeposited ZnO/Zn(OH)2 Nanosheets as a Functional Interface for Dendrite-Free Lithium Metal Anodes
Abstract
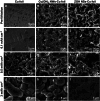
Modifying the current collector is a promising strategy to enable Li metal anodes with minimal Li consumption. Herein, a scalable electrodeposition method is introduced to construct 3D ZnO/Zn(OH)2 nanosheets on Cu foil (ZOH NSs-Cu foil). Cu(OH)2 nanowires are first formed via anodization, followed by electroconversion of Cu2+ and Zn2+ ions. DFT calculations reveal that the ZOH NSs-Cu foil exhibits high Li adsorption energy, imparting strong lithiophilicity and lowering the Li nucleation overpotential. The 3D nanosheet structure provides a large electrochemically active surface, reducing the effective current density. Furthermore, ZOH NSs-Cu foil exhibits low charge transfer resistance and promotes a Li2O/LiF-rich solid electrolyte interphase (SEI) layer, further reducing interfacial resistance. SEM analysis and simulations confirm uniform Li deposition on ZOH NSs-Cu foil. In asymmetric cells (1 mAh cm-2 at 1 mA cm-2), ZOH NSs-Cu foil supports stable cycling for over 400 cycles. Furthermore, a full cell coupling a LiFePO4 (LFP) cathode with a Li@ZOH NSs-Cu foil anode retains high capacity with ≈100% Coulombic efficiency over 350 cycles at 1 C, even at an N/P ratio of ≈1.9. This binder-free, scalable approach offers precise Li deposition control and excellent electrochemical performance, advancing the practical application of Li metal anodes.
Keywords: ZnO/Zn(OH)2 nanosheets; current collector modification; electrodeposition method; lithium metal anode; solid electrolyte interphases.
© 2025 The Author(s). Small published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Kim S., Park G., Lee S. J., Seo S., Ryu K., Kim C. H., Choi J. W., Adv. Mater. 2023, 35, 2206625. - PubMed
-
- Seo H. Y., Kim Y. B., Senthamaraikannan T. G., Lim D., Kang Y. C., Park G. D., ACS Nano 2025, 19, 6152. - PubMed
-
- Yang B., Hu A., Li T., Li K., Li Y., Jiang J., Xiao Z., Seh Z. W., Long J., Energy Storage Mater. 2024, 70, 103512.
-
- Wu X., Zhang S., Xu X., Wen F., Wang H., Chen H., Fan X., Huang N., Angew. Chem., Int. Ed. 2024, 63, 202319355. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

