Wearable Intervention for Alcohol Use Risk and Sleep in Young Adults: A Randomized Clinical Trial
- PMID: 40445615
- PMCID: PMC12125640
- DOI: 10.1001/jamanetworkopen.2025.13167
Wearable Intervention for Alcohol Use Risk and Sleep in Young Adults: A Randomized Clinical Trial
Abstract
Importance: Young adults in the US have the highest prevalence of alcohol use disorder; technology-based interventions may help to reduce drinking.
Objective: To test the efficacy of a multimodal digital intervention of wearable feedback and coaching for improving at-risk drinking and sleep health in young adults.
Design, setting, and participants: This parallel phase 2 randomized clinical trial was conducted from December 17, 2018, to May 19, 2021, at a research clinic in Connecticut. Participants were young adults (aged 18-25 years) from the local community (web and social media ads, public flyers) with sleep concerns, 3 or more heavy drinking occasions (≥5 drinks/occasion for men; ≥4 drinks/occasion for women) in the past 2 weeks, and a positive Alcohol Use Disorders Identification Test risk score. Analyses were conducted from November 10, 2023, to September 19, 2024, using an intention-to-treat approach.
Interventions: Wearable feedback and coaching plus web-based sleep advice plus smartphone self-monitoring or 1 of 2 control conditions, consisting of advice alone (control A) or advice plus self-monitoring (control A plus SM) for 2 weeks and follow-up to week 12. All participants wore sleep and alcohol biosensors.
Main outcomes and measures: The primary outcome consisted of total number of drinks in weeks 4 to 12. Secondary outcomes included sleep disturbance, sleep-related impairment, and alcohol-related consequences in weeks 4 to 12. An exploratory outcome was also assessed, reduction of 1 or more levels in World Health Organization (WHO) drinking risk from baseline to week 4. Models compared the wearable feedback and coaching with advice and self-monitoring with each control condition and changes from weeks 4 to 8 and 12 within each condition on baseline-adjusted outcomes.
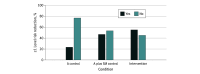
Results: A total of 120 participants were enrolled (61 [51%] women; 10 [8%] Asian; 9 [8%] Black; 19 [16%] Hispanic; 96 [80%] White; 1 [1%] multiracial; 4 [3%] other race or ethnicity), with a mean (SD) age of 21.16 (1.75) years. Sixty participants were randomized to the intervention, 30 to the control A group and 30 to the control A plus SM group. Total number of drinks (primary outcome) did not differ by condition or by condition × time, but number of drinks was significantly higher at weeks 4 vs 12 (49%) across conditions. For secondary outcomes, no condition effects were observed for drinking consequences and sleep disturbance, but sleep-related impairment and WHO risk-level reduction (exploratory outcome) differed by condition. Compared with the control A group, the intervention group reported clinically meaningful lower sleep-related impairment scores (mean [SE] least square mean difference, 3.09 [1.08]; 95% CI, 0.96-5.23) and were more than 3 times more likely to have reductions in WHO risk level (odds ratio, 3.85; 95% CI, 1.34-11.07; Cohen d = 0.72). Sleep disturbance improvement was associated with WHO risk-level reduction.
Conclusions and relevance: This randomized clinical trial did not detect a significant effect of the intervention on the primary outcome of total drinks or secondary outcomes of sleep disturbance or drinking consequences. The intervention significantly improved other measures of sleep health and drinking reduction compared with the control A condition and warrants further testing in larger samples.
Trial registration: ClinicalTrials.gov Identifier: NCT03658954.
Conflict of interest statement
Figures
References
-
- World Health Organization . Global status report on alcohol and health 2018. September 27, 2018. Accessed March 13, 2025. https://www.who.int/publications/i/item/9789241565639
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous