MTOR signaling regulates the development of airway mucous cell metaplasia associated with severe asthma
- PMID: 40446022
- PMCID: PMC12288895
- DOI: 10.1172/jci.insight.187904
MTOR signaling regulates the development of airway mucous cell metaplasia associated with severe asthma
Abstract
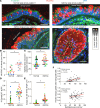
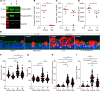
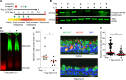
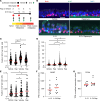
In asthma, airway epithelial remodeling is characterized by aberrant goblet cell metaplastic differentiation accompanied by epithelial cell hyperplasia and hypertrophy. These pathologic features in severe asthma indicate a loss of control of proliferation, cell size, differentiation, and migration. MTOR is a highly conserved pathway that regulates protein synthesis, cell size, and proliferation. We hypothesized that the balance between MTOR and autophagy regulates mucous cell metaplasia. Airways from individuals with severe asthma showed increased MTOR signaling by RPS6 phosphorylation, which was reproduced using an IL-13-activated model of primary human airway epithelial cells (hAEC). MTOR inhibition by rapamycin led to a decrease of IL-13-mediated cell hypertrophy, hyperplasia, and MUC5AC mucous metaplasia. BrdU labeling during IL-13-induced mucous metaplasia confirmed that MTOR was associated with increased basal-to-apical hAEC migration. MTOR activation by genetic deletion of Tsc2 in cultured mouse AECs increased IL-13-mediated hyperplasia, hypertrophy, and mucous metaplasia. Transcriptomic analysis of IL-13-stimulated hAEC identified MTOR-dependent expression of genes associated with epithelial migration and cytoskeletal organization. In summary, these findings point to IL-13-dependent and -independent roles of MTOR signaling in the development of pathogenic epithelial changes contributing to airway obstruction in severe asthma.
Keywords: Asthma; Autophagy; Cell biology; Cell migration/adhesion; Pulmonology.
Conflict of interest statement
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

