High-affinity PQQ import is widespread in Gram-negative bacteria
- PMID: 40446051
- PMCID: PMC12124388
- DOI: 10.1126/sciadv.adr2753
High-affinity PQQ import is widespread in Gram-negative bacteria
Abstract
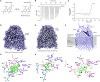
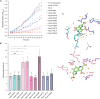
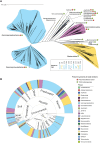
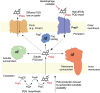
Pyrroloquinoline quinone (PQQ) is a soluble redox cofactor used by diverse bacteria. Many Gram-negative bacteria that encode PQQ-dependent enzymes do not produce it and instead obtain it from the environment. To achieve this, Escherichia coli uses the TonB-dependent transporter PqqU as a high-affinity PQQ importer. Here, we show that PqqU binds PQQ with high affinity and determine the high-resolution structure of the PqqU-PQQ complex, revealing that PqqU undergoes conformational changes in PQQ binding to capture the cofactor in an internal cavity. We show that these conformational changes preclude the binding of a bacteriophage, which targets PqqU as a cell surface receptor. Guided by the PqqU-PQQ structure, we identify amino acids essential for PQQ import and leverage this information to map the presence of PqqU across Gram-negative bacteria. This reveals that PqqU is encoded by Gram-negative bacteria from at least 22 phyla occupying diverse habitats, indicating that PQQ is an important cofactor for bacteria that adopt diverse lifestyles and metabolic strategies.
Figures


Similar articles
-
The TonB-dependent uptake of pyrroloquinoline-quinone (PQQ) and secretion of gluconate by Escherichia coli K-12.Mol Microbiol. 2022 Oct;118(4):417-425. doi: 10.1111/mmi.14975. Epub 2022 Sep 14. Mol Microbiol. 2022. PMID: 36054785
-
A Periplasmic Binding Protein for Pyrroloquinoline Quinone.Biochemistry. 2019 Jun 11;58(23):2665-2669. doi: 10.1021/acs.biochem.9b00358. Epub 2019 May 29. Biochemistry. 2019. PMID: 31140787
-
Crystal Structure of the Catalytic and Cytochrome b Domains in a Eukaryotic Pyrroloquinoline Quinone-Dependent Dehydrogenase.Appl Environ Microbiol. 2019 Nov 27;85(24):e01692-19. doi: 10.1128/AEM.01692-19. Print 2019 Dec 15. Appl Environ Microbiol. 2019. PMID: 31604769 Free PMC article.
-
Escherichia coli PQQ-containing quinoprotein glucose dehydrogenase: its structure comparison with other quinoproteins.Biochim Biophys Acta. 2003 Apr 11;1647(1-2):185-92. doi: 10.1016/s1570-9639(03)00100-6. Biochim Biophys Acta. 2003. PMID: 12686131 Review.
-
Biogenesis of the peptide-derived redox cofactor pyrroloquinoline quinone.Curr Opin Chem Biol. 2020 Dec;59:93-103. doi: 10.1016/j.cbpa.2020.05.001. Epub 2020 Jul 27. Curr Opin Chem Biol. 2020. PMID: 32731194 Free PMC article. Review.
References
-
- Anthony C., Pyrroloquinoline quinone (PQQ) and quinoprotein enzymes. Antioxid. Redox Signal. 3, 757–774 (2001). - PubMed
-
- Duine J. A., The PQQ story. J. Biosci. Bioeng. 88, 231–236 (1999). - PubMed
-
- Matsushita K., Toyama H., Yamada M., Adachi O., Quinoproteins: Structure, function, and biotechnological applications. Appl. Microbiol. Biotechnol. 58, 13–22 (2002). - PubMed
-
- Adachi O., Kubota T., Hacisalihoglu A., Toyama H., Shinagawa E., Duine J. A., Matsushita K., Characterization of quinohemoprotein amine dehydrogenase from Pseudomonas putida. Biosci. Biotechnol. Biochem. 62, 469–478 (1998). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

