Identification of a large anion channel required for digestive vacuole acidification and amino acid export in Plasmodium falciparum
- PMID: 40446075
- PMCID: PMC12158007
- DOI: 10.1371/journal.pbio.3003202
Identification of a large anion channel required for digestive vacuole acidification and amino acid export in Plasmodium falciparum
Abstract
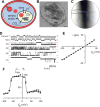
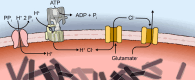
Malaria parasites survive in human erythrocytes by importing and digesting hemoglobin within a specialized organelle, the digestive vacuole (DV). Although chloroquine and other antimalarials act within the DV, the routes used by drugs, ions, and amino acids to cross the DV membrane remain poorly understood. Here, we used single DV patch-clamp to identify a novel large conductance anion channel as the primary conductive pathway on this organelle in Plasmodium falciparum, the most virulent human pathogen. This Big Vacuolar Anion Channel (BVAC) is primarily open at the DV resting membrane potential and undergoes complex voltage-dependent gating. Ion substitution experiments implicate promiscuous anion flux with Cl- being the primary charged substrate under physiological conditions. Conductance and gating are unaffected by antimalarials targeting essential DV activities and are conserved on parasites with divergent drug susceptibility profiles, implicating an unexploited antimalarial target. A conditional knockdown strategy excluded links to PfCRT and PfMDR1, two drug-resistance transporters with poorly defined transport activities. We propose that BVAC functions to maintain electroneutrality during H+ uptake, allowing DV acidification and efficient hemoglobin digestion. The channel also facilitates amino acid salvage, providing essential building blocks for parasite growth. Direct transport measurements at the DV membrane provide foundational insights into vacuolar physiology, should help clarify antimalarial action and drug resistance, and will guide therapy development against the parasite's metabolic powerhouse.
Copyright: This is an open access article, free of all copyright, and may be freely reproduced, distributed, transmitted, modified, built upon, or otherwise used by anyone for any lawful purpose. The work is made available under the Creative Commons CC0 public domain dedication.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



Similar articles
-
A 2-amino quinoline, 5-(3-(2-(7-chloroquinolin-2-yl)ethenyl)phenyl)-8-dimethylcarbamyl-4,6-dithiaoctanoic acid, interacts with PfMDR1 and inhibits its drug transport in Plasmodium falciparum.Mol Biochem Parasitol. 2014 Jun;195(1):34-42. doi: 10.1016/j.molbiopara.2014.05.006. Epub 2014 Jun 8. Mol Biochem Parasitol. 2014. PMID: 24914817
-
Mechanistic basis for multidrug resistance and collateral drug sensitivity conferred to the malaria parasite by polymorphisms in PfMDR1 and PfCRT.PLoS Biol. 2022 May 4;20(5):e3001616. doi: 10.1371/journal.pbio.3001616. eCollection 2022 May. PLoS Biol. 2022. PMID: 35507548 Free PMC article.
-
Structural and evolutionary analyses of the Plasmodium falciparum chloroquine resistance transporter.Sci Rep. 2020 Mar 16;10(1):4842. doi: 10.1038/s41598-020-61181-1. Sci Rep. 2020. PMID: 32179795 Free PMC article.
-
The malaria digestive vacuole.Front Biosci (Schol Ed). 2012 Jun 1;4(4):1424-48. doi: 10.2741/s344. Front Biosci (Schol Ed). 2012. PMID: 22652884 Review.
-
Plasmodium's bottomless pit: properties and functions of the malaria parasite's digestive vacuole.Trends Parasitol. 2022 Jul;38(7):525-543. doi: 10.1016/j.pt.2022.02.010. Epub 2022 Mar 19. Trends Parasitol. 2022. PMID: 35317985 Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

