Microinvasive Probes for Monitoring Electrical and Chemical Neural Activity in Nonhuman Primates
- PMID: 40446210
- PMCID: PMC12183687
- DOI: 10.1021/acschemneuro.5c00071
Microinvasive Probes for Monitoring Electrical and Chemical Neural Activity in Nonhuman Primates
Abstract
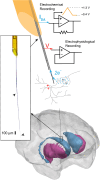
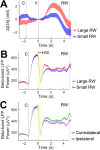
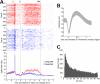
We leveraged carbon fiber materials for creating sensors that provide dual neurochemical and electrical neural activity recording at microinvasive (10 μm) spatial footprints proximal to recording sites, and enabling these measurements from deep brain targets of primates with conventional cranial chambers. These shaft-assisted microinvasive probes (s-μIPs) are approximately 10 μm in diameter along the distal length (1-15 mm) immediately surrounding the targeted recording site. This microinvasive portion ensures that the recording site is isolated from tissue damage induced by the wider shaft portion of the device. The shaft (150-165 μm in diameter) within the device stiffens the remaining length of the probe (>100 mm), and provides compatibility with standard intracranial insertion protocols (e.g., guide tubes and chamber setups) that require a sufficiently rigid and long shaft for deep brain insertion in monkeys. The s-μIP was further expanded to provide dual-channel chemical and electrical neural activity recording with micrometer spatial resolution. Measurements of reward- and movement- related dopamine, spikes, and local field potentials were made from single and dual-channel s-μIPs implanted in task-performing monkeys. Recordings from chronically implanted s-μIPs display the capability of functional multimodal (chemical and electrical) neural activity measurements over 1-year postimplantation from microinvasive devices.
Keywords: dopamine recording; fast-scan cyclic voltammetry; microinvasive neural implant.
Figures


Update of
-
Micro-invasive probes for monitoring electrical and chemical neural activity in nonhuman primates.bioRxiv [Preprint]. 2025 Feb 5:2025.01.30.635139. doi: 10.1101/2025.01.30.635139. bioRxiv. 2025. Update in: ACS Chem Neurosci. 2025 Jun 18;16(12):2237-2247. doi: 10.1021/acschemneuro.5c00071. PMID: 39975231 Free PMC article. Updated. Preprint.
Similar articles
-
Micro-invasive probes for monitoring electrical and chemical neural activity in nonhuman primates.bioRxiv [Preprint]. 2025 Feb 5:2025.01.30.635139. doi: 10.1101/2025.01.30.635139. bioRxiv. 2025. Update in: ACS Chem Neurosci. 2025 Jun 18;16(12):2237-2247. doi: 10.1021/acschemneuro.5c00071. PMID: 39975231 Free PMC article. Updated. Preprint.
-
Transcutaneous electrical nerve stimulation (TENS) for fibromyalgia in adults.Cochrane Database Syst Rev. 2017 Oct 9;10(10):CD012172. doi: 10.1002/14651858.CD012172.pub2. Cochrane Database Syst Rev. 2017. PMID: 28990665 Free PMC article.
-
Interventions for eye movement disorders due to acquired brain injury.Cochrane Database Syst Rev. 2018 Mar 5;3(3):CD011290. doi: 10.1002/14651858.CD011290.pub2. Cochrane Database Syst Rev. 2018. PMID: 29505103 Free PMC article.
-
The measurement and monitoring of surgical adverse events.Health Technol Assess. 2001;5(22):1-194. doi: 10.3310/hta5220. Health Technol Assess. 2001. PMID: 11532239
-
Large-scale high-density brain-wide neural recording in nonhuman primates.Nat Neurosci. 2025 Jul;28(7):1562-1575. doi: 10.1038/s41593-025-01976-5. Epub 2025 Jun 23. Nat Neurosci. 2025. PMID: 40551025 Free PMC article.
References
-
- Schwerdt H. N., Zhang E., Kim M. J., Yoshida T., Stanwicks L., Amemori S., Dagdeviren H. E., Langer R., Cima M. J., Graybiel A. M.. Cellular-scale probes enable stable chronic subsecond monitoring of dopamine neurochemicals in a rodent model. Commun. Biol. 2018;1:144. doi: 10.1038/s42003-018-0147-y. - DOI - PMC - PubMed
-
- Schwerdt H. N., Shimazu H., Amemori K., Amemori S., Tierney P. L., Gibson D. J., Hong S., Yoshida T., Langer R., Cima M. J., Graybiel A. M.. Long-term dopamine neurochemical monitoring in primates. Proc. Natl. Acad. Sci. U.S.A. 2017;114:13260–13265. doi: 10.1073/pnas.1713756114. - DOI - PMC - PubMed
-
- Luan L., Wei X., Zhao Z., Siegel J. J., Potnis O., Tuppen C. A., Lin S., Kazmi S., Fowler R. A., Holloway S., Dunn A. K., Chitwood R. A., Xie C.. Ultraflexible nanoelectronic probes form reliable, glial scar–free neural integration. Sci. Adv. 2017;3:e1601966. doi: 10.1126/sciadv.1601966. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

